Impact and Value of Hospital Antibiotic Stewardship: Retrospective Pre-COVID-19-Pandemic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intervention
2.2. Outcomes
2.3. Statistical Analysis
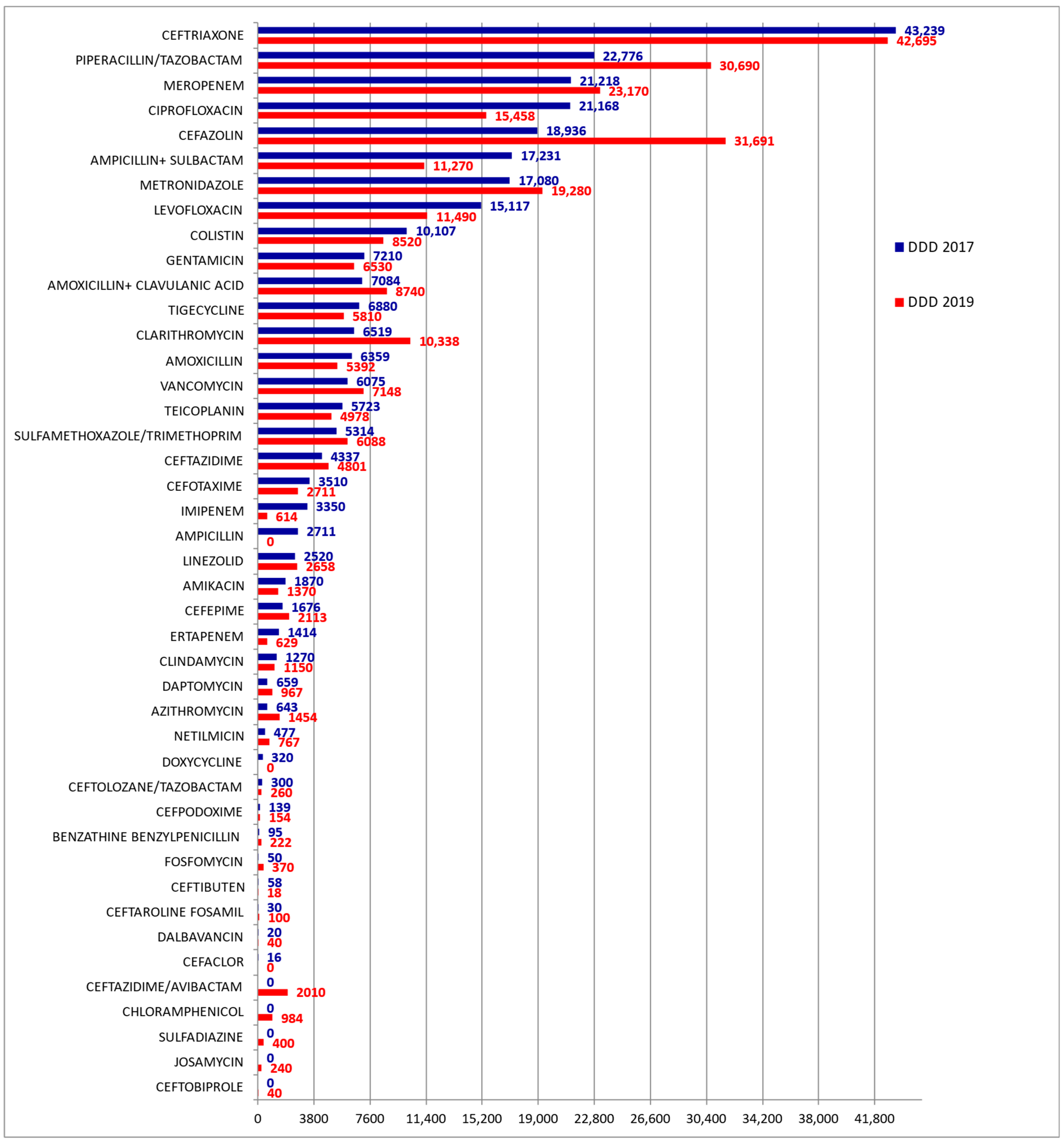
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunha, C.B. Antimicrobial Stewardship Programs: Principles and Practice. Med. Clin. N. Am. 2018, 102, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.; Cioffi, G.; Moccia, G.; Filippelli, A.; De Caro, F. Stewardship per l’utilizzo degli antibiotici. Una nuova strategia per il controllo dell’antibiotico-resistenza. In La Sanità Pubblica: Ricerca Applicata, 3rd ed.; Giuseppe De Nicola Publisher: Naples, Italy, 2021; pp. 11–48. [Google Scholar]
- Parente, D.M.; Morton, J. Role of the Pharmacist in Antimicrobial Stewardship. Med. Clin. N. Am. 2018, 102, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N. The search for good antimicrobial stewardship. Jt. Comm. J. Qual. Improv. 2001, 27, 403–404. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrave, S.E. Antimicrobial Stewardship. Infect. Dis. Clin. 2011, 25, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Costantino, M.; Mangrella, M.; D’Orsi, G.; Orlando, G.; Parlato, A.; Rossi, F. Antibiotic therapy for respiratory infection treatment. Ig. Mod. 1999, 112, 2001–2014. [Google Scholar]
- Ranise, A.; Bondavalli, F.; Bruno, O.; Schenone, S.; Losasso, C.; Costantino, M.; Cenicola, M.L.; Donnoli, D.; Marmo, E. 3,3-Disubstituted 1-acyl-1-phenylthioureas with platelet antiaggregating and other activities. Farmaco 1991, 46, 317–338. [Google Scholar] [PubMed]
- Antibiotic Drugs. Available online: https://www.aifa.gov.it/en/web/guest/farmaci-antibiotici (accessed on 4 July 2022).
- European Centre for Disease Prevention and Control (ECDC). Antimi-crobial consumption. In ECDC Annual Epidemiological Report for 2018; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Conti, V.; Sellitto, C.; Torsiello, M.; Manzo, V.; De Bellis, E.; Stefanelli, B.; Bertini, N.; Costantino, M.; Maci, C.; Raschi, E.; et al. Identification of Drug Interaction Adverse Events in Patients with COVID-19: A Systematic Review. JAMA Netw. Open 2022, 5, e227970. [Google Scholar] [CrossRef] [PubMed]
- Transatlantic Taskforce on Antimicrobial Resistance (TAFTAR). Available online: https://www.cdc.gov/drugresistance/tatfar/about.html (accessed on 1 May 2022).
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. The Healthcare-Associated Infections Prevalence Study Group. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Eur. Surveill 2018, 23, 1800516. [Google Scholar] [CrossRef] [Green Version]
- Permanent Conference for Relations between the State, the Regions and the Autonomous Provinces of Trento and Bolzano—PNCAR 2017–2020, Italian National Plan to Combat Antibiotic Resistance–November 2017. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2660 (accessed on 20 March 2022).
- Bellino, S.; Iacchini, S.; Monaco, M.; Del Grosso, M.; Camilli, R.; Errico, G.; Giufrè, M.; Sisi, S.; D’Ancona, F.P.; Pantosti, A.; et al. AR-ISS: Sorveglianza Nazionale dell’Antibiotico-Resistenza- Rapporti ISS Sorveglianza RIS-1/2021. Available online: Iss.it/documents/20126/o/RIS-1-2021.pdf (accessed on 10 March 2022).
- Casillo, M.; Castiglione, A.; Colace, F.; De Santo, M.; Marongiu, F.; Santaniello, D. COVID-19 data sharing and organization through blockchain and decentralized models. In Proceedings of the 2021 Joint Business Informatics Research Workshops and Doctoral Consortium, BIR-WS 2021, Vienna, Austria, 22–24 September 2021; Volume 2991, pp. 128–140. [Google Scholar]
- The European House-Ambrosetti S.p.A. Rapporto Meridiano Sanità. 2017. Available online: https://www.sanita24.ilsole24ore.com/pdf2010/Editrice/ILSOLE24ORE/QUOTIDIANO_SANITA/Online/_Oggetti_Correlati/Documenti/2017/11/15/Rapporto_MS2017.pdf?uuid=AEKFj6AD (accessed on 3 April 2022).
- Special Eurobarometer 445 “Antimicrobial Resistance”. 2016. Available online: https://www.jpiamr.eu/app/uploads/2016/06/EUbarometer_SpecialReportonAMR_Summary.pdf (accessed on 15 April 2022).
- Censis. Italians and Antibiotics: Information, Use and Awareness of the Antimicrobial-Resistance Phenomenon, Rome. 2020. Available online: https://www.censis.it/sites/default/files/downloads/rapporto_finale_antibiotici.pdf (accessed on 12 May 2022).
- AMR Review Paper—Tackling a Crisis for the Health and Wealth of Nations, Chaired by Jim O’Neill December 2014. Available online: https://www.google.com/search?client=safari&rls=en&q=MR+Review+Paper%E2%80%94Tackling+a+crisis+for+the+health+and+wealth+of+nations,+Chaired+by+Jim+O%E2%80%99Neill+December+2014.&ie=UTF-8&oe=UTF-8 (accessed on 1 March 2022).
- Mantaldo, C.; Lanini, S.; Curiale, S.; Ippolito, G. An innovative strategy to evaluate new antibiotics in times of antibiotic-resistance. GImPIOS 2019, 9, 96–100. [Google Scholar]
- Costantino, M.; Sellitto, C.; Conti, V.; Corbi, G.; Marongiu, F.; Genovese, G.; Moccia, G.; Capunzo, M.; Borrelli, A.; Pagliano, P.; et al. Adverse Events Associated with BNT162b2 and AZD1222 Vaccines in the Real World: Surveillance Report in a Single Italian Vaccine Center. J. Clin. Med. 2022, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Antimictobial Stewardship. Available online: https://www.sifoweb.it/images/pdf/pubblicazioni/altre-edizioni/antimicrobial-stewardship/Antimicrobial_Stewardship_in_medicina.pdf (accessed on 20 July 2022).
- Department of Health of the Campania Region Directorate General for Health Protection and Coordination of the Regional Health System. Report 2019 on Antibiotic Resistance and the Use of Antibiotics Detected in Public Structures of the Health System of Campania. Available online: http://www.regione.campania.it/regione/it/tematiche/antibiotico-resistenza-ed-infezionicorrelate-all-assistenza-64in (accessed on 21 March 2022).
- Available online: http://www.mattoni.ministerosalute.it (accessed on 20 March 2022).
- Italian National Plan to Combat Antibiotic Resistance (PNCAR) 2017–2020. 2017. Available online: www.salute.gov.it (accessed on 3 June 2022).
- Antimicrobial Resistance in the EU/EEA. A One Health Response. Available online: https://www.oecd.org (accessed on 15 April 2022).
- Malta, R.; Di Rosa, S.; D’Alessandro, N. Ethical aspects in the management of antibacterial agents utilization. Ital. J. Med. 2010, 4, 137–144. [Google Scholar] [CrossRef]
- ATC/DDD Index 2022. Available online: http://www.whocc.no/atc_ddd_ (accessed on 2 May 2022).
- AR-ISS: National Surveillance of the Antibiotic-Resistance. Available online: https://www.iss.it/documents/20126/0/RIS-1_2021.pdf/af6da4cc-0f57-3800-68ca-c5f6c05479c0?t=1637230397225 (accessed on 10 April 2022).
- Addressing Antimicrobial Resistance: Progress in the Animal Sector, but This Health Threat Remains a Challenge for the EU. Available online: https://op.europa.eu/webpub/eca/special-reports/amr-18-2019/it/2019 (accessed on 4 April 2022).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, P56–P66. [Google Scholar] [CrossRef] [Green Version]
- CAESAR and EARS-Net Surveillance Data. Excutive Summary of the 2020 Data on Antimicrobial Resistance in Europe. 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/348971/9789289056298-eng.pdf (accessed on 30 April 2022).
- Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containingmedicinal-products (accessed on 21 July 2022).
- Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2017. Available online: https://farmaci.agenziafarmaco.gov.it/bancadatifarmaci/ (accessed on 21 July 2022).
- Last-Line Antibiotics Are Failing: Options to Address This Urgent Threat to Patients and Healthcare Systems. Available online: https://www.ecdc.europa.eu/sites/default/files/media/it/publications/Publications/antibiotic-resistance-policy-briefing.pdf (accessed on 21 July 2022).
- Costantino, M. The rhinogenic deafness and SPA therapy: Clinical-experimental study. Clin. Ter. 2008, 159, 311–315. [Google Scholar] [PubMed]
- Antimicrobial Resistance (AMR): Thinking the Impossible. Available online: Aifa.gov.it/documents/20142/15805co/abstract-seminario_1_IT.pdf (accessed on 29 March 2022).
- Tomas, A.; Pavlović, N.; Stilinović, N.; Horvat, O.; Paut-Kusturica, M.; Dugandžija, T.; Tomić, Z.; Sabo, A. Increase and Change in the Pattern of Antibiotic Use in Serbia (2010–2019). Antibiotics 2021, 10, 397. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Time Period (Years) | % Variation | |
|---|---|---|---|
| 2017 | 2019 | ||
| DDD of antibiotics consumed | 263,501 | 273,360 | +3.7 |
| Costs | 1,072,083 | 783,682 | −26.9 |
| DDD/100 bed-days | 107 | 112 | +4.7 |
| DRG weight means | 1.26 | 1.29 | +2.4 |
| Average cost of therapy per day | 4.1 | 2.9 | −29.3 |
| Antibiotic Category | DDD Consumed in 2017 | DDD Consumed in 2019 | p | Δ% in 2017 vs. 2019 |
|---|---|---|---|---|
| Fluoroquinolones, n(%) | 36,285 (13.8) | 26,948 (9.9) | <0.05 | −25.7 |
| Carbapenems, n(%) | 25,982 (9.9) | 24,413 (8.9) | <0.05 | −6.0 |
| Cephalosporins, n(%) | 72,241 (27.4) | 86,593 (31.7) | <0.05 | +19.9 |
| Macrolides, n(%) | 7162 (2.7) | 12,032 (4.4) | <0.05 | +68.0 |
| Polymyxins, n(%) | 10,107 (3.8) | 8520 (3.1) | <0.05 | −15.7 |
| Lincosamides, n(%) | 1270 (0.48) | 1150 (0.42) | <0.05 | −9.4 |
| AWaRe Category | DDD Consumed in 2017 | DDD Consumed in 2019 | p | Δ% in 2017 vs. 2019 |
|---|---|---|---|---|
| ACCESS, n(%) | 85,480 (32.4) | 93,117 (34.1) | <0.05 | +8.9 |
| WATCH, n(%) | 157,505 (59.8) | 159,838 (58.5) | <0.05 | +1.5 |
| RESERVE, n(%) | 20,516 (7.8) | 20,405 (7.5) | <0.05 | −0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantino, M.; Conti, V.; Corbi, G.; Iannelli, A.A.; Marongiu, F.; Torsiello, M.; Della Vecchia, A.; Sellitto, C.; Genovese, A.; Moccia, G.; et al. Impact and Value of Hospital Antibiotic Stewardship: Retrospective Pre-COVID-19-Pandemic Analysis. J. Clin. Med. 2022, 11, 4412. https://doi.org/10.3390/jcm11154412
Costantino M, Conti V, Corbi G, Iannelli AA, Marongiu F, Torsiello M, Della Vecchia A, Sellitto C, Genovese A, Moccia G, et al. Impact and Value of Hospital Antibiotic Stewardship: Retrospective Pre-COVID-19-Pandemic Analysis. Journal of Clinical Medicine. 2022; 11(15):4412. https://doi.org/10.3390/jcm11154412
Chicago/Turabian StyleCostantino, Maria, Valeria Conti, Graziamaria Corbi, Alessandra Anna Iannelli, Francesco Marongiu, Martina Torsiello, Antonio Della Vecchia, Carmine Sellitto, Armando Genovese, Giuseppina Moccia, and et al. 2022. "Impact and Value of Hospital Antibiotic Stewardship: Retrospective Pre-COVID-19-Pandemic Analysis" Journal of Clinical Medicine 11, no. 15: 4412. https://doi.org/10.3390/jcm11154412
APA StyleCostantino, M., Conti, V., Corbi, G., Iannelli, A. A., Marongiu, F., Torsiello, M., Della Vecchia, A., Sellitto, C., Genovese, A., Moccia, G., Filippelli, A., & De Caro, F. (2022). Impact and Value of Hospital Antibiotic Stewardship: Retrospective Pre-COVID-19-Pandemic Analysis. Journal of Clinical Medicine, 11(15), 4412. https://doi.org/10.3390/jcm11154412






