Propranolol: A “Pick and Roll” Team Player in Benign Tumors and Cancer Therapies
Abstract
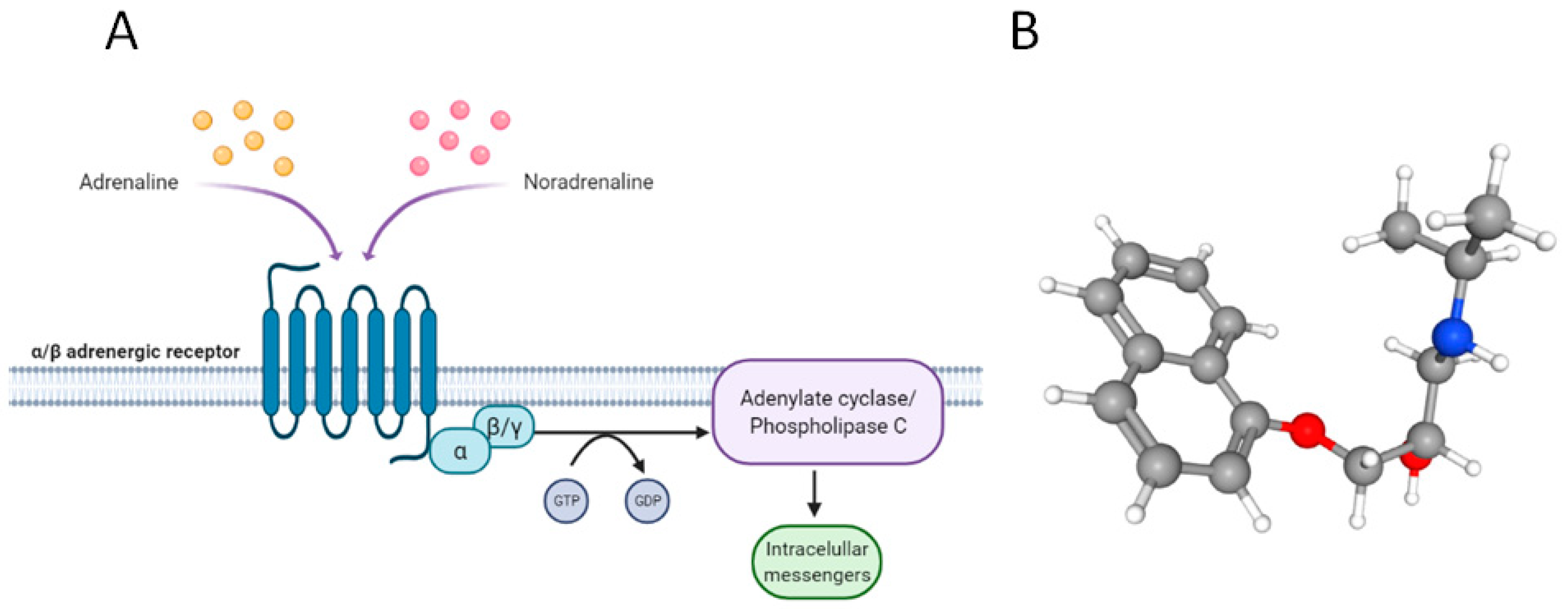
:1. Introduction
2. Why Propranolol?
2.1. Vasoconstriction
2.2. Inhibition of Angiogenesis
2.3. Induction of Apoptosis
3. Propranolol as Adjuvant Therapy at Single Administration in Clinical Trials
3.1. Infantile Hemangioma
3.2. Propranolol for Solid Tumors
Preoperative Propranolol in Breast Cancer
3.3. Propranolol for Non-Solid Tumors
Multiple Myeloma
3.4. Propranolol in Observational Studies
4. Propranolol in Combinatorial Therapy
4.1. Propranolol and Etodolac (COX-2 Inhibitor)
4.1.1. Breast Cancer
4.1.2. Colorectal Cancer
4.1.3. Pancreatic Cancer
4.1.4. Neuroblastoma
4.2. Propranolol and Prednisolone (Corticosteroid)
4.3. Propranolol and Standard Chemotherapy
4.3.1. Hepatocellular Carcinoma (HCC)
4.3.2. Ovarian Cancer
4.3.3. Breast Cancer
4.3.4. Esophageal Adenocarcinoma
4.3.5. Sarcoma and Melanoma
4.3.6. Bladder Cancer
4.3.7. Diverse Tumors
5. Conclusions
- -
- The ADBR1-2 antagonist propranolol has emerged as a candidate treatment for several tumor processes. Although its mechanism of action has yet to be investigated, it is proposed as an adjuvant drug at single administration or in combined therapies due to its safety profile and therapeutic experience that support its use.
- -
- The potential therapeutic value of propranolol is supported by its intervention in physiological and molecular mechanisms, such as vasodilatation, apoptosis, or angiogenesis.
- -
- Propranolol was tested in several clinical trials for IH at different doses, the timing of treatment, and comparing it to current pharmacological treatments of IH prior to propranolol. In almost all the cases, propranolol showed therapeutic benefits and no side effects.
- -
- The EMA gave the marketing authorization to Hemangiol (oral propranolol) after a multicenter phase 3 clinical trial for the treatment of IH. Afterward, propranolol has also been used in monotherapy and combination therapy in different clinical trials to treat different types of tumors.
- -
- Among the solid tumors other than IH, the use of propranolol to prevent tumor dissemination and reduce metastasis as a preoperative treatment prior to surgeries in breast, prostate, and ovarian cancer, stands out.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockaert, J.; Pin, J.P. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilardaga, J.P.; Bünemann, M.; Feinstein, T.N.; Lambert, N.; Nikolaev, V.O.; Engelhardt, S.; Lohse, M.J.; Hoffmann, C. Minireview: GPCR and G proteins: Drug efficacy and activation in live cells. Mol. Endocrinol. 2009, 23, 590–599. [Google Scholar] [CrossRef]
- Arang, N.; Gutkind, J.S. G Protein-Coupled receptors and heterotrimeric G proteins as cancer drivers. FEBS Lett. 2020, 594, 4201–4232. [Google Scholar] [CrossRef]
- Black, J.W.; Crowther, A.F.; Shanks, R.G.; Smith, L.H.; Dornhorst, A.C. A new adrenergic. Beta-receptor antagonist. Lancet 1964, 1, 1080–1081. [Google Scholar] [CrossRef]
- Olesen, J.; Hertz, M. Isoproterenol and propranolol: Ability to cross the blood-brain barrier and effects on cerebral circulation in man. Stroke 1978, 9, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlquist, R.P. A Study of the Adrenotropic Receptors. Am. J. Physiol. 1948, 153, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.P. Sir James Black and Propranolol the Role of the Basic Sciences in the History of Cardiovascular Pharmacology. Tex. Heart Inst. J. 1997, 24, 336–342. [Google Scholar]
- Prichard, B.N.C.; Ross, E.J. Use of propranolol in conjunction with alpha receptor blocking drugs in pheochromocytoma. Am. J. Cardiol. 1966, 18, 394–398. [Google Scholar] [CrossRef]
- Boréus, L.O.; Broberger, U.; Nergårdh, A.; Zetterqvist, P. Malignant pheochromocytoma in a child: Treatment with a combination of alpha- and beta-adrenergic blockade. Acta Paediatr. 1968, 57, 36–40. [Google Scholar] [CrossRef]
- Scharf, Y.; Nahir, A.M.; Better, O.S.; Koten, A.; Arieh, Y.B.; Gellei, B. Prolonged survival in malignant pheochromocytoma of the organ of zuckerkandl with pharmacological treatment. Cancer 1973, 31, 746–750. [Google Scholar] [CrossRef]
- Yoshida, S.; Hatori, M.; Noshiro, T.; Kimura, N.; Kokubun, S. Twenty-six-years’ survival with multiple bone metastasis of malignant pheochromocytoma. Arch. Orthop. Trauma. Surg. 2001, 121, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.B.; Perkins, J.P. Regulation of Adenosine 3’:5’-Cyclic Monophosphate Concentration in Cultured Human Astrocytoma Cells by Catecholamines and Histamine. Proc. Natl. Acad. Sci. USA 1971, 68, 2757–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, L.A.; Butcher, R.W. Studies on Cyclic AMP Metabolism in Human Epidermoid Carcinoma (HEp-2) Cells. Metabolism 1975, 24, 359–368. [Google Scholar] [CrossRef]
- Delavier-Klutchko, C.; Hoebeke, J.; Strosberg, A.D. The Human Carcinoma Cell Line A431 Possesses Large Numbers of Functional β-Adrenergic Receptors. FEBS Lett. 1984, 169, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Blum, I.; Rusecki, Y.; Doron, M.; Lahav, M.; Laron, Z.; Atsmon, A. Evidence for a Therapeutic Effect of Dl-Propranolol in Benign and Malignant Insulinoma: Report of Three Cases. J. Endocrinol. Investig. 1983, 6, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Cole, B. Regulation of cell proliferation by β-adrenergjc receptors in a human lung adenocarcinoma cell line. Carcinogenesis 1989, 10, 1753–1755. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Zhang, J.; Dancel, R.; Garcia, S.J.; Willis, C.; Seidler, F.J. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2000, 60, 153–166. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; de la Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for Severe Hemangiomas of Infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Storch, C.H.; Hoeger, P.H. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br. J. Dermatol. 2010, 163, 269–274. [Google Scholar] [CrossRef]
- Sánchez-Carpintero, I.; Ruiz-Rodriguez, R.; López-Gutiérrez, J.C. Propranolol in the treatment of infantile hemangioma: Clinical effectiveness, risks, and recommendations. Actas Dermo-Sifiliográficas 2011, 102, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Léauté-Labrèze, C.; Hoeger, P.; Mazereeuw-Hautier, J.; Guibaud, L.; Baselga, E.; Posiunas, G.; Phillips, R.J.; Caceres, H.; Lopez Gutierrez, J.C.; Ballona, R.; et al. A Randomized, Controlled Trial of Oral Propranolol in Infantile Hemangioma. N. Engl. J. Med. 2015, 372, 735–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquier, E.; Ciccolini, J.; Carre, M.; Giacometti, S.; Fanciullino, R.; Pouchy, C.; Montero, M.P.; Serdjebi, C.; Kavallaris, M.; André, N. Propranolol potentiates the anti-angiogenic effects and antitumor efficacy of chemotherapy agents: Implication in breast cancer treatment. Oncotarget 2011, 2, 797–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masur, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 2001, 61, 2866–2869. [Google Scholar] [PubMed]
- Liao, X.; Che, X.; Zhao, W.; Zhang, D.; Bi, T.; Wang, G. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol. Rep. 2010, 24, 1669–1676. [Google Scholar] [PubMed] [Green Version]
- Kim, H.S.; Park, Y.H.; Lee, H.S.; Kwon, M.J.; Song, J.H.; Chang, I.B. Propranolol inhibits the proliferation of human glioblastoma cell lines through notch1 and hes1 signaling system. J. Korean Neurosurg. Soc. 2021, 64, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Porter, B.; Riechert, A. Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J. Cancer Res. Clin. Oncol. 2000, 126, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Park, P.G.; Merryman, J.; Orloff, M.; Schuller, H.M. β-Adrenergic Mitogenic Signal Transduction in Peripheral Lung Adenocarcinoma: Implications for Individuals with Preexisting Chronic Lung Disease. Cancer Res. 1995, 55, 3504–3508. [Google Scholar]
- Yang, E.V.; Sood, A.K.; Chen, M.; Li, Y.; Eubank, T.D.; Marsh, C.B.; Jewell, S.; Flavahan, N.A.; Morrison, C.; Yeh, P.E.; et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006, 66, 10357–10364. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.K.; Bhatty, R.; Kamat, A.A.; Landen, C.N.; Han, L.; Thaker, P.H.; Li, Y.; Gershenson, D.M.; Lutgendorf, S.; Cole, S.W. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 2006, 12, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.G.; Morizono, K.; Karanikolas, B.D.W.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Ma, Q.; Shen, S.; Hu, H. Inhibition of Pancreatic Cancer Cell Proliferation by Propranolol Occurs Through Apoptosis Induction. Pancreas 2009, 38, 94–100. [Google Scholar] [CrossRef]
- Al-Wadei, H.A.; Al-Wadei, M.H.; Schuller, H.M. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs 2009, 20, 477–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Ma, Q.Y.; Hu, H.T.; Zhang, M. β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol. Ther. 2010, 10, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westfall, T.C.; Westfall, D.P. Neurotransmission: The autonomic and somatic motor nervous systems. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Westfall, T.C.; Westfall, D.P. Adrenergic agonists and antagonists. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Sommers Smith, S.K.; Smith, D.M. Beta blockade induces apoptosis in cultured capillary endothelial cells. Vitr. Cell Dev. Biol. Anim. 2002, 38, 298–304. [Google Scholar] [CrossRef]
- Annabi, B.; Lachambre, M.P.; Plouffe, K.; Moumdjian, R.; Béliveau, R. Propranolol Adrenergic Blockade Inhibits Human Brain Endothelial Cells Tubulogenesis and Matrix Metalloproteinase-9 Secretion. Pharmacol. Res. 2009, 60, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Lachambre, M.P.; Lord-Dufour, S.; Béliveau, R. Propranolol Suppresses Angiogenesis in Vitro: Inhibition of Proliferation, Migration, and Differentiation of Endothelial Cells. Vasc. Pharmacol. 2010, 53, 200–208. [Google Scholar] [CrossRef]
- Sharifpanah, F.; Saliu, F.; Bekhite, M.M.; Wartenberg, M.; Sauer, H. β-Adrenergic receptor antagonists inhibit vasculogenesis of embryonic stem cells by downregulation of nitric oxide generation and interference with VEGF signalling. Cell Tissue Res. 2014, 358, 443–452. [Google Scholar] [CrossRef]
- Chim, H.; Armijo, B.S.; Miller, E.; Gliniak, C.; Serret, M.A.; Gosain, A.K. Propranolol Induces Regression of Hemangioma Cells through HIF-1α-Mediated Inhibition of VEGF-A. Ann. Surg. 2012, 256, 146–156. [Google Scholar] [CrossRef]
- Pan, W.K.; Li, P.; Guo, Z.T.; Huang, Q.; Gao, Y. Propranolol Induces Regression of Hemangioma Cells via the Down-Regulation of the PI3K/Akt/ENOS/VEGF Pathway. Pediatr. Blood Cancer 2015, 62, 1414–1420. [Google Scholar] [CrossRef]
- Zhang, L.; Mai, H.M.; Zheng, J.; Zheng, J.W.; Wang, Y.A.; Qin, Z.P.; Li, K.L. Propranolol Inhibits Angiogenesis via Down-Regulating the Expression of Vascular Endothelial Growth Factor in Hemangioma Derived Stem Cell. Int. J. Clin. Exp. Pathol. 2013, 7, 48–55. [Google Scholar] [PubMed]
- Munabi, N.C.; England, R.W.; Edwards, A.K.; Kitajewski, A.A.; Tan, Q.K.; Weinstein, A.; Kung, J.E.; Wilcox, M.; Kitajewski, J.K.; Shawber, C.J.; et al. Propranolol Targets Hemangioma Stem Cells via cAMP and Mitogen-Activated Protein Kinase Regulation. Stem Cells Transl. Med. 2016, 5, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.; Hardy, K.L.; Kitajewski, A.M.; Shawber, C.J.; Kitajewski, J.K.; Wu, J.K. Propranolol accelerates adipogenesis in hemangioma stem cells and causes apoptosis of hemangioma endothelial cells. Plast. Reconstr. Surg. 2012, 130, 1012–1021. [Google Scholar] [CrossRef]
- Albiñana, V.; Villar Gómez De Las Heras, K.; Serrano-Heras, G.; Segura, T.; Perona-Moratalla, A.B.; Mota-Pérez, M.; de Campos, J.M.; Botella, L.M. Propranolol Reduces Viability and Induces Apoptosis in Hemangioblastoma Cells from von Hippel-Lindau Patients. Orphanet J. Rare Dis. 2015, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, A.M.; Albiñana, V.; Gallardo-Vara, E.; Recio-Poveda, L.; de Rojas-P, I.; de Las Heras, K.V.G.; Aguirre, D.T.; Botella, L.M. The Β2-Adrenergic Receptor Antagonist ICI-118,551 Blocks the Constitutively Activated HIF Signalling in Hemangioblastomas from von Hippel-Lindau Disease. Sci. Rep. 2019, 9, 10062. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Street, J.; Pouchy, C.; Carre, M.; Gifford, A.J.; Murray, J.; Norris, M.D.; Trahair, T.; Andre, N.; Kavallaris, M. B-Blockers Increase Response to Chemotherapy via Direct Antitumour and Anti-Angiogenic Mechanisms in Neuroblastoma. Br. J. Cancer 2013, 108, 2485–2494. [Google Scholar] [CrossRef] [Green Version]
- Hajighasemi, F.; Hajighasemi, S. Effect of Propranolol on Angiogenic Factors in Human Hematopoietic Cell Lines in Vitro. Iran. Biomed. J. 2009, 13, 223–228. [Google Scholar]
- Ristori, C.; Filippi, L.; dal Monte, M.; Martini, D.; Cammalleri, M.; Fortunato, P.; la Marca, G.; Fiorini, P.; Bagnoli, P. Role of the Adrenergic System in a Mouse Model of Oxygen-Induced Retinopathy: Antiangiogenic Effects of β-Adrenoreceptor Blockade. Investig. Ophthalmol. Vis. Sci. 2011, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, K.M.; Chang, K.W.; Truong, M.T.; Kwok, S.; West, R.B.; Heerema-Mckenney, A.E. β-Adrenergic Receptor Expression in Vascular Tumors. Mod. Pathol. 2012, 25, 1446–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.Y.; He, R.H.; Zhang, J.; He, Y.J.; Wan, Z.; Zhou, C.F.; Tang, Y.J.; Li, Z.; McLeod, H.L.; Liu, J. β-blockers inhibit the viability of breast cancer cells by regulating the ERK/COX-2 signaling pathway and the drug response is affected by ADRB2 single-nucleotide polymorphisms. Oncol. Rep. 2019, 41, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Albiñana, V.; Escribano, R.M.J.; Soler, I.; Padial, L.R.; Recio-Poveda, L.; Villar Gómez De Las Heras, K.; Botella, L.M. Repurposing propranolol as a drug for the treatment of retinal haemangioblastomas in von Hippel-Lindau disease. Orphanet J. Rare Dis. 2017, 12, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albiñana, V.; Gallardo-Vara, E.; de Rojas-P, I.; Recio-Poveda, L.; Aguado, T.; Canto-Cano, A.; Aguirre, D.T.; Serra, M.M.; González-Peramato, P.; Martínez-Piñeiro, L.; et al. Targeting β2-Adrenergic Receptors Shows Therapeutical Benefits in Clear Cell Renal Cell Carcinoma from Von Hippel–Lindau Disease. J. Clin. Med. 2020, 9, 2740. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, B.; De Las Heras, K.V.G.; Aguirre, D.T.; Rodríguez-Padial, L.; Albiñana, V.; Recio-Poveda, L.; Cuesta, A.M.; Botella, L.M.; Jiménez-Escribano, R.M. Evaluation of the safety and effectiveness of oral propranolol in patients with von Hippel-Lindau disease and retinal hemangioblastomas: Phase III clinical trial. BMJ Open Ophthalmol. 2019, 4, e000203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Léauté-Labrèze, C.; Dumas De La Roque, E.; Nacka, F.; Abouelfath, A.; Grenier, N.; Rebola, M.; Ezzedine, K.; Moore, N. Double-blind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br. J. Dermatol. 2013, 169, 181–183. [Google Scholar]
- Hogeling, M.; Adams, S.; Wargon, O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics 2011, 128, e259-66. [Google Scholar] [CrossRef] [Green Version]
- Baselga, E.; Dembowska-Baginska, B.; Przewratil, P.; González-Enseñat, M.A.; Wyrzykowski, D.; Torrelo, A.; Gutiérrez, J.C.L.; Rychłowska-Pruszynska, M.; De Lucas-Laguna, R.; Esteve-Martinez, A.; et al. Efficacy of propranolol between 6 and 12 months of age in high-risk infantile hemangioma. Pediatrics 2018, 142, e20173866. [Google Scholar] [CrossRef] [Green Version]
- Bauman, N.M.; McCarter, R.J.; Guzzetta, P.C.; Shin, J.J.; Oh, A.K.; Preciado, D.A.; He, J.; Greene, E.A.; Puttgen, K.B. Propranolol vs prednisolone for symptomatic proliferating infantile hemangiomas: A randomized clinical trial. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Choi, T.H.; Choi, Y.; Park, Y.W.; Hong, K.Y.; Kim, D.Y.; Choe, Y.S.; Lee, H.; Cheon, J.E.; Park, J.B.; et al. Comparison of efficacy and safety between propranolol and steroid for infantile hemangioma: A randomized clinical trial. JAMA Dermatol. 2017, 153, 529–536. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, S.; Yang, K.; Zhang, X.; Zhou, J.; Li, L.; Xiang, B.; Qiu, T.; Dai, S.; Jiang, X.; et al. Efficacy and Safety of Propranolol vs Atenolol in Infants with Problematic Infantile Hemangiomas: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 599–607. [Google Scholar] [CrossRef]
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT03237637 (accessed on 14 June 2022).
- Socchi, F.; Bigorre, M.; Normandin, M.; Captier, G.; Bessis, D.; Mondain, M.; Blanchet, C.; Akkari, M.; Amedro, P.; Gavotto, A. Hemangiol in infantile haemangioma: A paediatric post-marketing surveillance drug study. Br. J. Clin. Pharmacol. 2021, 87, 1970–1980. [Google Scholar] [CrossRef]
- Hiller, J.G.; Cole, S.W.; Crone, E.M.; Byrne, D.J.; Shackleford, D.M.; Pang, J.M.B.; Henderson, M.A.; Nightingale, S.S.; Ho, K.M.; Myles, P.S.; et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: A phase II randomized trial. Clin. Cancer Res. 2020, 26, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Spini, A.; Roberto, G.; Gini, R.; Bartolini, C.; Bazzani, L.; Donnini, S.; Crispino, S.; Ziche, M. Evidence of β-blockers drug repurposing for the treatment of triple negative breast cancer: A systematic review. Neoplasma 2019, 66, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Varela-Ramirez, A.; Dickerson, E.; Pasquier, E.; Torabi, A.; Aguilera, R.; Nahleh, Z.; Bryan, B. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed. J. 2019, 42, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.M.; Kerswill, S.A.; Hari, P.; Cole, S.W.; Logan, B.R.; D’Souza, A.; Shah, N.N.; Horowitz, M.M.; Stolley, M.R.; Sloan, E.K.; et al. Repurposing existing medications as cancer therapy: Design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer 2018, 18, 593. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.M.; Rizzo, J.D.; Hari, P.; Pasquini, M.C.; Giles, K.E.; D’Souza, A.; Logan, B.R.; Hamadani, M.; Chhabra, S.; Dhakal, B.; et al. Propranolol inhibits molecular risk markers in HCT recipients: A phase 2 randomized controlled biomarker trial. Blood Adv. 2020, 4, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Benish, M.; Bartal, I.; Goldfarb, Y.; Levi, B.; Avraham, R.; Raz, A.; Ben-Eliyahu, S. Perioperative use of β-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann. Surg. Oncol. 2008, 15, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J. Norepinephrine release may play a critical role in the warburg effect: An integrative model of tumorigenesis. Neoplasma 2020, 67, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Scheff, N.N.; Saloman, J.L. Neuroimmunology of cancer and associated symptomology. Immunol. Cell Biol. 2021, 99, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Jara-Gutiérrez, Á.; Baladrón, V. The role of prostaglandins in different types of cancer. Cells 2021, 10, 1487. [Google Scholar] [CrossRef]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving Survival Rates in Two Models of Spontaneous Postoperative Metastasis in Mice by Combined Administration of a β-Adrenergic Antagonist and a Cyclooxygenase-2 Inhibitor. J. Immunol. 2010, 184, 2449–2457. [Google Scholar] [CrossRef] [Green Version]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving Postoperative Immune Status and Resistance to Cancer Metastasis. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Qi, M.; Peng, C.; Zhou, C.; Su, J.; Zeng, W.; Liu, H.; Zhang, J.; Chen, M.; Shen, M.; et al. Propranolol enhanced the anti-tumor effect of sunitinib by inhibiting proliferation and inducing G0/G1/S phase arrest in malignant melanoma. Oncotarget 2017, 9, 802–811. [Google Scholar] [CrossRef] [Green Version]
- Kokolus, K.M.; Zhang, Y.; Sivik, J.M.; Schmeck, C.; Zhu, J.; Repasky, E.A.; Drabick, J.J.; Schell, T.D. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 2017, 7, e1405205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico, M.; Baglioni, M.; Bondarenko, M.; Laluce, N.C.; Rozados, V.; André, N.; Carré, M.; Scharovsky, O.G.; Márquez, M.M. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget 2017, 8, 2874–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brohée, L.; Peulen, O.; Nusgens, B.; Castronovo, V.; Thiry, M.; Colige, A.C.; Deroanne, C.F. Propranolol sensitizes prostate cancer cells to glucose metabolism inhibition and prevents cancer progression. Sci. Rep. 2018, 8, 7050. [Google Scholar] [CrossRef] [PubMed]
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT02013492 (accessed on 14 June 2022).
- Haldar, R.; Shaashua, L.; Lavon, H.; Lyons, Y.A.; Zmora, O.; Sharon, E.; Birnbaum, Y.; Allweis, T.; Sood, A.K.; Barshack, I.; et al. Perioperative inhibition of β-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav. Immun. 2018, 73, 294–309. [Google Scholar] [CrossRef]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 2017, 23, 4651–4661. [Google Scholar] [CrossRef] [Green Version]
- Haldar, R.; Ricon-Becker, I.; Radin, A.; Gutman, M.; Cole, S.W.; Zmora, O.; Ben-Eliyahu, S. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: A randomized controlled trial. Cancer 2020, 126, 3991–4001. [Google Scholar] [CrossRef]
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT00888797 (accessed on 14 June 2022).
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT03919461 (accessed on 14 June 2022).
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT03838029 (accessed on 14 June 2022).
- Berthold, F.; Hömberg, M.; Proleskovskaya, I.; Mazanek, P.; Belogurova, M.; Ernst, A.; Sterba, J. Metronomic therapy has low toxicity and is as effective as current standard treatment for recurrent high-risk neuroblastoma. Pediatr. Hematol. Oncol. 2017, 34, 308–319. [Google Scholar] [CrossRef]
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01074437 (accessed on 14 June 2022).
- NIH, US National Library of Medicine. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01265576 (accessed on 14 June 2022).
- Ramondetta, L.M.; Hu, W.; Thaker, P.H.; Urbauer, D.L.; Chisholm, G.B.; Westin, S.N.; Sun, Y.; Ramirez, P.T.; Fleming, N.; Sahai, S.K.; et al. Prospective pilot trial with combination of propranolol with chemotherapy in patients with epithelial ovarian cancer and evaluation on circulating immune cell gene expression. Gynecol. Oncol. 2019, 154, 524–530. [Google Scholar] [CrossRef]
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT04682158 (accessed on 14 June 2022).
- Hopson, M.B.; Lee, S.; Accordino, M.; Trivedi, M.; Maurer, M.; Crew, K.D.; Hershman, D.L.; Kalinsky, K. Phase II study of propranolol feasibility with neoadjuvant chemotherapy in patients with newly diagnosed breast cancer. Breast Cancer Res. Treat. 2021, 188, 427–432. [Google Scholar] [CrossRef] [PubMed]
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT03384836 (accessed on 14 June 2022).
- NIH, US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT04493489 (accessed on 14 June 2022).



| Drug in Combination | Therapeutic Effect | Type of Cancer | Ref |
|---|---|---|---|
| Bacilli Calmette-Guerin | Immune System activator | Bladder | [93] |
| Captopril | Angiotensin-converting enzyme (ACE) inhibitor | Infantile Hemangioma | * |
| Carboplatin | DNA duplication interferent | Esophageal Adenocarcinoma Fallopian Tube Invasive Epithelial Ovarian Primary Peritoneal | [89,90] |
| Celecoxib cyclophosphamide | COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID) | Neuroblastoma | [86] |
| Cilazapril | Angiotensin-converting enzyme (ACE) inhibitor | Glioblastoma Head and Neck skin Squamous Cell Metastatic Melanoma Oral cavity Squamous Cell | * |
| Cyclophosphamide | Alkylating agent | Breast | [91] |
| Doxorubicin | DNA duplication interferent | Breast | [91] |
| Etodolac | Nonsteroidal anti-inflammatory drug (NSAID) | Breast Colorectal Pancreatic Neuroblastoma | [79,80,81,82,83,84,85] |
| Etoposide | Topoisomerase II inhibitor | Neuroblastoma | * |
| Losartan | Angiotensin II receptor antagonist | Glioblastoma Head and Neck skin Squamous cell carcinoma Metastatic Melanoma Oral cavity Squamous Cell | * |
| Metformin | Inhibitor of the mitochondrial respiratory chain (complex I) | Glioblastoma Head and Neck skin Squamous Cell Metastatic Melanoma Oral cavity Squamous Cell | * |
| Paclitaxel | Mitotic inhibitor | Breast Esophageal Adenocarcinoma Fallopian Tube Invasive Epithelial Ovarian Primary Peritoneal | [89,90,91] |
| Pegfilgrastim | Stimulate the production of neutrophils | Breast | [91] |
| Pembrolizumab | Binds to and blocks PD-1 | Cutaneous Melanoma | [92] |
| Pertuzumab | HER2 dimerization inhibitor | Breast | [91] |
| Piperine | Alkaloid | Glioblastoma Head and Neck skin Squamous Cell Metastatic Melanoma Oral cavity Squamous Cell | * |
| Prednisolone | Immunosuppressor | Kaposiform Hemangioendothelioma Kasabach Merritt Phenomenon | * |
| Sirolimus | Immunosuppressor | Kaposiform Hemangioendothelioma Kasabach Merritt Phenomenon | * |
| Trastuzumab | HER2 antagonist | Breast | [91] |
| Vinblastine | Microtubules assembly inhibitor | Neuroblastoma | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albiñana, V.; Gallardo-Vara, E.; Casado-Vela, J.; Recio-Poveda, L.; Botella, L.M.; Cuesta, A.M. Propranolol: A “Pick and Roll” Team Player in Benign Tumors and Cancer Therapies. J. Clin. Med. 2022, 11, 4539. https://doi.org/10.3390/jcm11154539
Albiñana V, Gallardo-Vara E, Casado-Vela J, Recio-Poveda L, Botella LM, Cuesta AM. Propranolol: A “Pick and Roll” Team Player in Benign Tumors and Cancer Therapies. Journal of Clinical Medicine. 2022; 11(15):4539. https://doi.org/10.3390/jcm11154539
Chicago/Turabian StyleAlbiñana, Virginia, Eunate Gallardo-Vara, Juan Casado-Vela, Lucía Recio-Poveda, Luisa María Botella, and Angel M Cuesta. 2022. "Propranolol: A “Pick and Roll” Team Player in Benign Tumors and Cancer Therapies" Journal of Clinical Medicine 11, no. 15: 4539. https://doi.org/10.3390/jcm11154539








