Complete Right Bundle Branch Block as a Predictor of Cardiovascular Events in Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Exposure and Outcome
2.3. Other Variables
2.4. Statistical Analysis
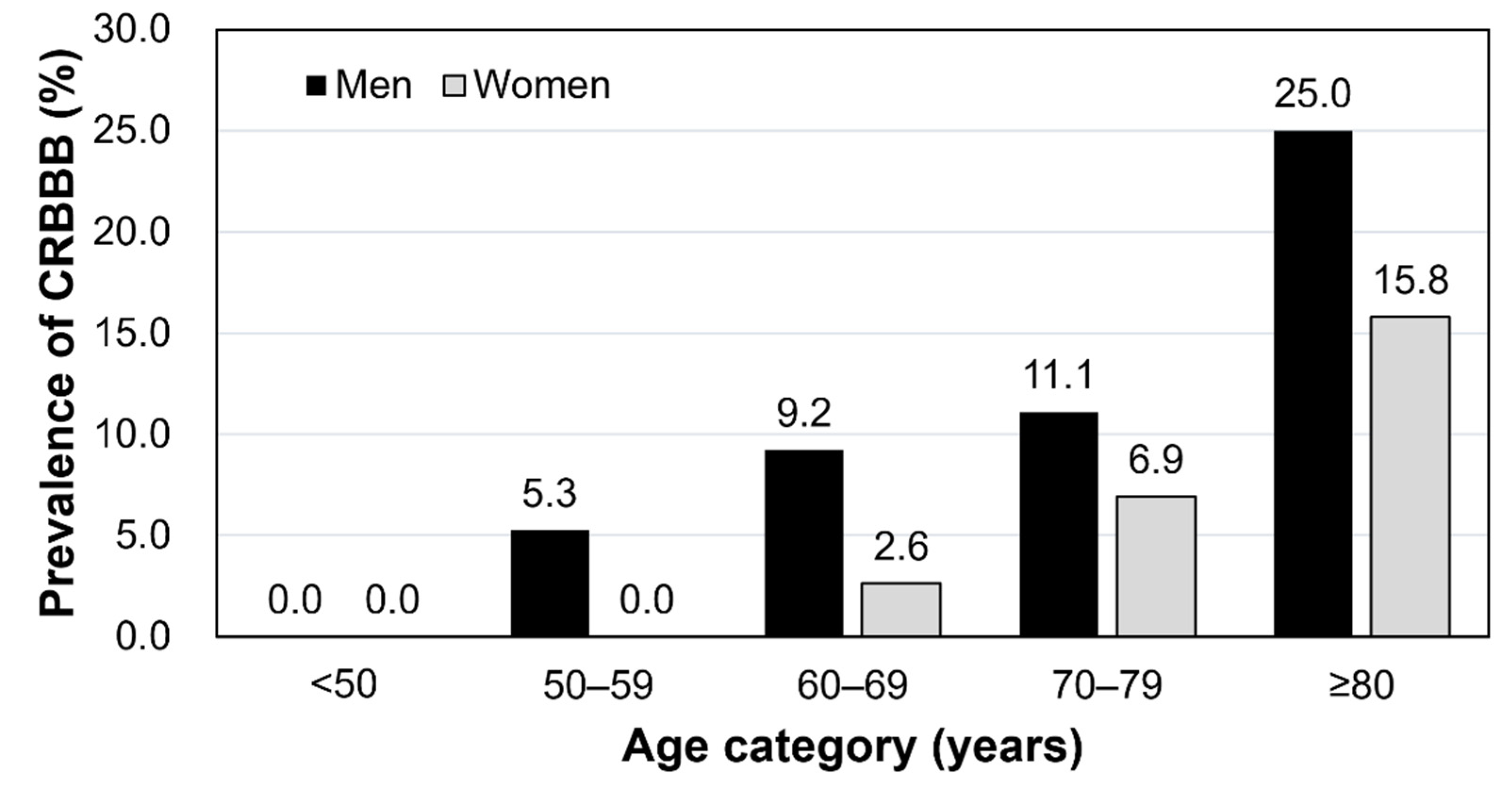
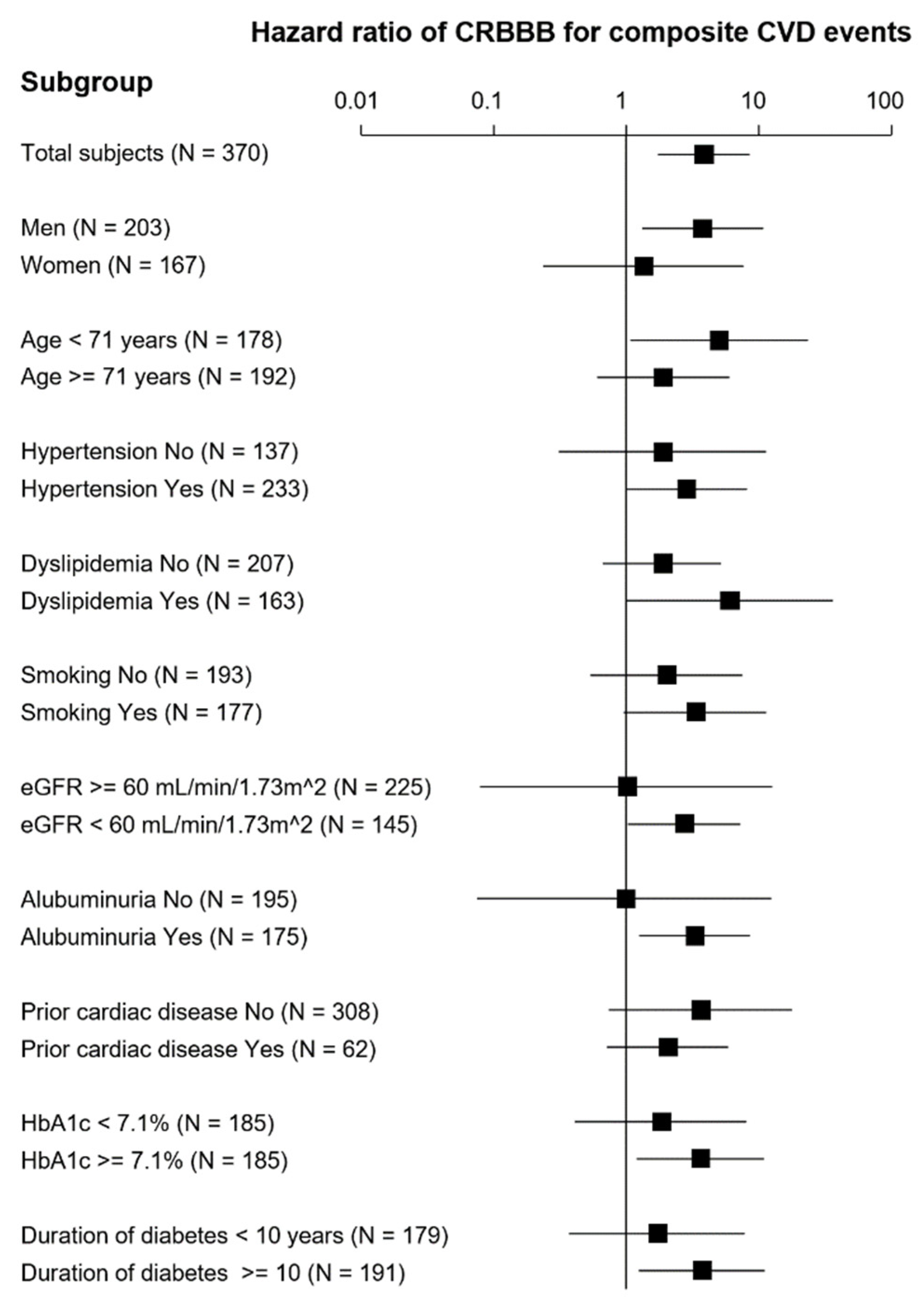
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleg, J.L.; Das, D.N.; Lakatta, E.G. Right bundle branch block: Long-Term prognosis in apparently healthy men. J. Am. Coll. Cardiol. 1983, 1, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Fahy, G.J.; Pinski, S.L.; Miller, D.P.; McCabe, N.; Pye, C.; Walsh, M.J.; Robinson, K. Natural history of isolated bundle branch block. Am. J. Cardiol. 1996, 77, 1185–1190. [Google Scholar] [CrossRef]
- Eriksson, P.; Hansson, P.O.; Eriksson, H.; Dellborg, M. Bundle-branch block in a general male population: The study of men born 1913. Circulation 1998, 98, 2494–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W.L.; Hodge, D.O.; Hammill, S.C. Association of Uncomplicated Electrocardiographic Conduction Blocks with Subsequent Cardiac Morbidity in a Community-Based Population (Olmsted County, Minnesota). Am. J. Cardiol. 2008, 101, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.S.-F.; Chen, C.-Y.J.; Wu, I.-C.; Hsu, C.-C.; Chen, T.-Y.; Tseng, W.-T.; Tang, F.-C.; Wang, C.-C.; Juan, C.-C.; Chiu, H.-C.; et al. Prognostic value and prevalence of complete right bundle branch block in an elderly population: A community-based 10-year prospective study. Aging 2020, 12, 19073–19082. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, L.; Liu, W.; Hankey, G.; Xu, B.; Wang, S. The Prognostic Significance of Right Bundle Branch Block: A Meta-analysis of Prospective Cohort Studies. Clin. Cardiol. 2015, 38, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhong, A.; You, T.; Chen, J.; Xu, W.; Shi, M. Prognostic Significance of Right Bundle Branch Block for Patients with Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Bussink, B.E.; Holst, A.G.; Jespersen, L.; Deckers, J.W.; Jensen, G.B.; Prescott, E. Right bundle branch block: Prevalence, risk factors, and outcome in the general population: Results from the Copenhagen City Heart Study. Eur. Heart J. 2013, 34, 138–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaba, P.; Pedrotty, D.; DeSimone, C.V.; Bonikowske, A.R.; Allison, T.G.; Kapa, S. Mortality in Patients With Right Bundle-Branch Block in the Absence of Cardiovascular Disease. J. Am. Heart Assoc. 2020, 9, e017430. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, J.H.; Park, Y.H.; Han, D.C.; Hwang, K.W.; Lee, D.W.; Oh, J.H.; Song, S.G.; Kim, J.S.; Chun, K.J.; et al. Incidence of and Risk Factors for Bundle Branch Block in Adults older than 40 years. Korean J. Intern. Med. 2004, 19, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Sowers, J.R.; Smith, S.C. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamoto, K.; Ando, K.; Fujita, T.; Hasebe, N.; Higaki, J.; Horiuchi, M.; Umemura, S.; Imai, Y.; Ito, M.; Itoh, H.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens. Res. 2014, 37, 253–390. [Google Scholar] [PubMed] [Green Version]
- Kinoshita, M.; Yokote, K.; Arai, H.; Iida, M.; Ishigaki, Y.; Ishibashi, S.; Umemoto, S.; Egusa, G.; Ohmura, H.; Okamura, T.; et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J. Atheroscler. Thromb. 2018, 25, 846–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Total | CRBBB (–) | CRBBB (+) | p Value |
|---|---|---|---|---|
| Number | 370 | 336 | 34 | --- |
| Age (years) | 71 (64–77) | 71 (64–76) | 75 (69–81) | 0.004 |
| Female sex N (%) | 167 (45.1%) | 155 (46.1%) | 12 (35.3%) | 0.226 |
| Pre-existing heart disease N (%) | 76 (20.5%) | 68 (20.2%) | 8 (23.5%) | 0.658 |
| Coronary heart disease N (%) | 28 (7.6%) | 27 (8.0%) | 1 (2.9%) | 0.495 |
| Cardiomyopathy N (%) | 9 (2.4%) | 8 (2.4%) | 1 (2.9%) | 0.584 |
| Left ventricular hypertrophy N (%) | 6 (1.6%) | 5 (1.5%) | 1 (2.9%) | 0.442 |
| Valvular heart disease N (%) | 14 (3.8%) | 12 (3.6%) | 2 (5.9%) | 0.375 |
| Left ventricular diastolic disorder N (%) | 3 (0.8%) | 3 (0.9%) | 0 (0.0%) | 1.000 |
| Congenital heart disease N (%) | 3 (0.8%) | 3 (0.9%) | 0 (0.0% | 1.000 |
| Atrial fibrillation N (%) | 27 (7.3%) | 22 (6.6%) | 5 (14.7%) | 0.089 |
| Current smoker N (%) | 177 (47.8%) | 161 (47.9%) | 16 (47.1%) | 0.924 |
| Hypertension N (%) | 233 (63.0%) | 211 (62.8%) | 22 (64.7%) | 0.826 |
| Dyslipidemia N (%) | 163 (44.1%) | 152 (45.2%) | 11 (32.4%) | 0.149 |
| eGFR (mL/min/1.73 m2) | 65.0 (54.7–77.5) | 65.4 (55.5–77.8) | 59.7 (45.4–67.2) | 0.017 |
| Albuminuria (normo/micro/overt) N (%) | 195/110/65 (52.7%/29.7%/17.6%) | 180/101/55 (53.6%/30.1%/16.4%) | 15/9/10 (44.1%/26.5%/29.4%) | 0.161 |
| BMI (kg/m2) | 23.8 (21.3–26.9) | 23.9 (21.3–26.9) | 22.7 (21.2–26.9) | 0.343 |
| HbA1c (%) | 7.1 (6.5–8.1) | 7.1 (6.5–8.1) | 6.9 (6.3–8.1) | 0.206 |
| Duration of T2DM | 10 (5–16) | 10 (4–16) | 9 (5–22) | 0.628 |
| SBP (mmHg) | 138 (124–149) | 138 (124–150) | 137 (127–143) | 0.347 |
| DBP (mmHg) | 75 (66–84) | 75 (67–84) | 72 (65–77) | 0.035 |
| TC (mg/dL) | 190 (165–213) | 191 (165–213) | 185 (158–207) | 0.340 |
| LDL-C (mg/dL) | 110 (91–131) | 110 (91–132) | 110 (80–129) | 0.488 |
| HDL-C (mg/dL) | 55 (44–66) | 55 (45–66) | 52 (41–66) | 0.713 |
| Triglyceride (mg/dL) | 115 (78–162) | 115 (78–166) | 113 (89–142) | 0.669 |
| Medications | ||||
| Anticoagulants | 25 (6.8%) | 21 (6.3%) | 4 (11.8%) | 0.222 |
| RASi N (%) | 186 (50.3%) | 169 (50.3%) | 17 (50.0%) | 0.974 |
| CCB N (%) | 155 (41.9%) | 145 (43.2%) | 10 (29.4%) | 0.122 |
| β-blocker N (%) | 36 (9.7%) | 35 (10.4%) | 1 (2.9%) | 0.161 |
| MRA N (%) | 15 (4.1%) | 13 (3.9%) | 2 (5.9%) | 0.571 |
| Statin N (%) | 159 (43.0%) | 146 (43.5%) | 13 (38.2%) | 0.558 |
| Insulin N (%) | 73 (19.7%) | 68 (20.2%) | 5 (14.7%) | 0.440 |
| SGLT2i N (%) | 5 (1.4%) | 5 (1.5%) | 0 (0%) | 0.474 |
| GLP-1 RA N (%) | 2 (0.5%) | 2 (0.6%) | 0 (0%) | 0.652 |
| Biguanide N (%) | 89 (24.1%) | 85 (25.3%) | 4 (11.8%) | 0.079 |
| DPP4i N (%) | 229 (61.9%) | 210 (62.5%) | 19 (55.9%) | 0.449 |
| Sulfonylurea N (%) | 139 (37.6%) | 130 (38.7%) | 9 (26.5%) | 0.161 |
| Thiazolidine N (%) | 31 (8.4%) | 30 (8.93%) | 1 (2.94%) | 0.230 |
| Glinide N (%) | 5 (1.4%) | 4 (1.20%) | 1 (2.94%) | 0.400 |
| α-GI N (%) | 56 (15.1%) | 52 (15.5%) | 4 (11.8%) | 0.565 |
| CRBBB (–) | CRBBB (+) | |||
|---|---|---|---|---|
| Number of patients | 312 | 26 | ||
| Number of cases | 24 | 8 | ||
| Patient-years | 1598 | 106 | ||
| Crude rate per 1000 patient-years | 16.3 | 75.6 | ||
| Model | Adjustment | HR (95% CI) by Fine–Gray model | p value | |
| 1 | Crude | 1.00 (Reference) | 3.85 (1.74–8.53) | 0.001 |
| 2 | IPTW method | 1.00 (Reference) | 2.55 (1.04–6.26) | 0.041 |
| 3 | IPTW method + covariates adjustment * | 1.00 (Reference) | 3.05 (1.30–7.13) | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, K.; Uchimoto, S.; Miyazaki, M.; Honda, N.; Mori, K.; Morioka, T.; Imai, T.; Shoji, T.; Emoto, M. Complete Right Bundle Branch Block as a Predictor of Cardiovascular Events in Type 2 Diabetes. J. Clin. Med. 2022, 11, 4618. https://doi.org/10.3390/jcm11154618
Ono K, Uchimoto S, Miyazaki M, Honda N, Mori K, Morioka T, Imai T, Shoji T, Emoto M. Complete Right Bundle Branch Block as a Predictor of Cardiovascular Events in Type 2 Diabetes. Journal of Clinical Medicine. 2022; 11(15):4618. https://doi.org/10.3390/jcm11154618
Chicago/Turabian StyleOno, Katsuhiro, Sadahiko Uchimoto, Masamune Miyazaki, Natsuki Honda, Katsuhito Mori, Tomoaki Morioka, Takumi Imai, Tetsuo Shoji, and Masanori Emoto. 2022. "Complete Right Bundle Branch Block as a Predictor of Cardiovascular Events in Type 2 Diabetes" Journal of Clinical Medicine 11, no. 15: 4618. https://doi.org/10.3390/jcm11154618
APA StyleOno, K., Uchimoto, S., Miyazaki, M., Honda, N., Mori, K., Morioka, T., Imai, T., Shoji, T., & Emoto, M. (2022). Complete Right Bundle Branch Block as a Predictor of Cardiovascular Events in Type 2 Diabetes. Journal of Clinical Medicine, 11(15), 4618. https://doi.org/10.3390/jcm11154618






