Predictive Modeling for Suicide-Related Outcomes and Risk Factors among Patients with Pain Conditions: A Systematic Review
Abstract
:1. Introduction
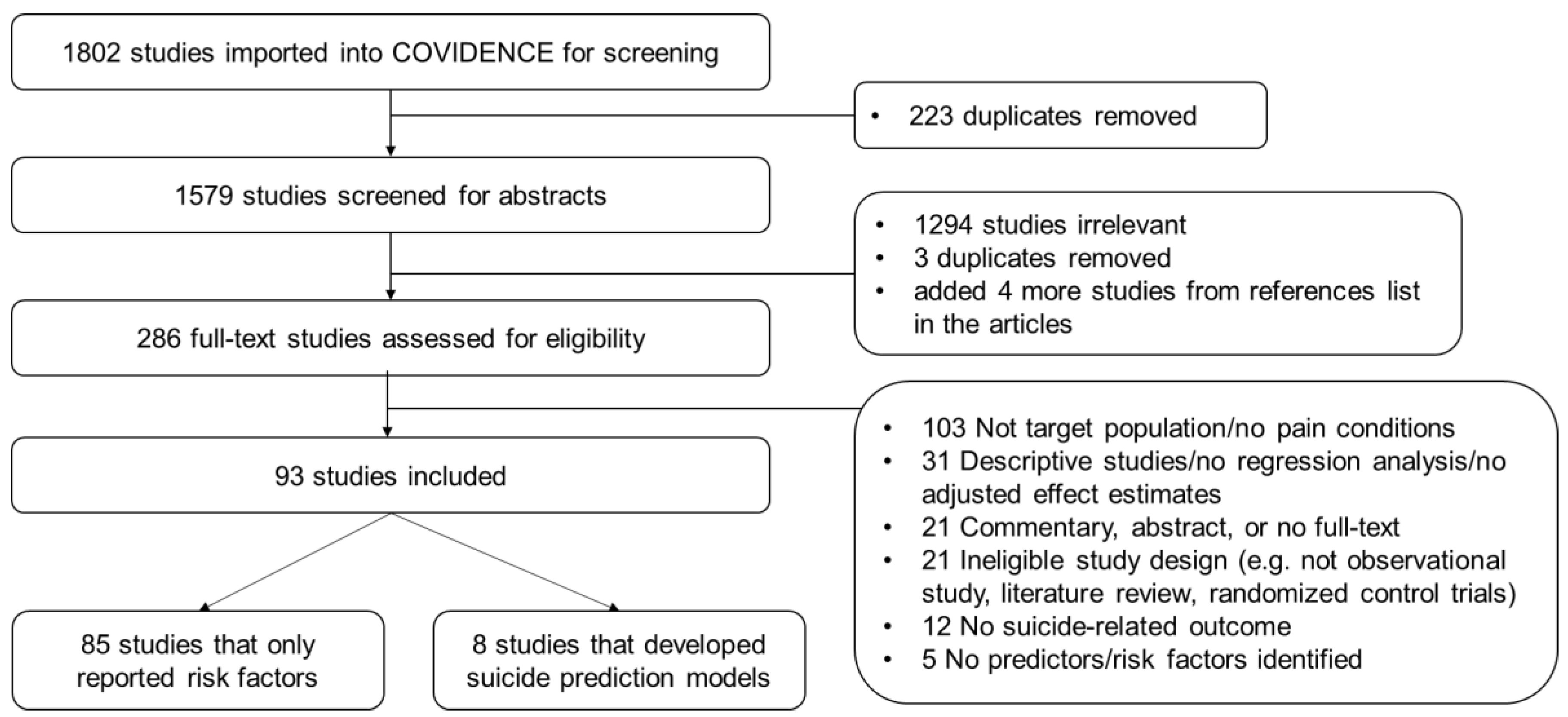
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Study Selection and Data Extraction
2.3. Risk of Bias Assessment
2.4. Study Outcomes of Interest
3. Results
3.1. Characteristics of Included Studies
3.2. Factors Associated with Suicide-Related Outcomes among Patients with Pain Conditions
3.3. Performance of Studies Developing Suicide Prediction Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS). 2003. Available online: https://webappa.cdc.gov/sasweb/ncipc/leadcause.html (accessed on 1 August 2022).
- Hedegaard, H.; Curtin, S.C.; Warner, M. Increase in Suicide Mortality in the United States, 1999–2018; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8. [Google Scholar]
- National Institute of Mental Health. Suicide is a Leading Cause of Death in the United States. 2021. Available online: https://www.nimh.nih.gov/health/statistics/suicide.shtml (accessed on 1 August 2022).
- Breslau, N. Migraine, suicidal ideation, and suicide attempts. Neurology 1992, 42, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Rigatti-Luchini, S.; Fracca, F.; Merskey, H. Suicidality in chronic abdominal pain: An analysis of the Hispanic Health and Nutrition Examination Survey (HHANES). Pain 1998, 76, 137–144. [Google Scholar] [CrossRef]
- Ashrafioun, L.; Bishop, T.M.; Conner, K.R.; Pigeon, W.R. Frequency of prescription opioid misuse and suicidal ideation, planning, and attempts. J. Psychiatr. Res. 2017, 92, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R. Risk Factors for Opioid-Use Disorder and Overdose. Anesth. Analg. 2017, 125, 1741–1748. [Google Scholar] [CrossRef]
- Campbell, G.; Bruno, R.; Darke, S.; Shand, F.; Hall, W.; Farrell, M.; Degenhardt, L. Prevalence and Correlates of Suicidal Thoughts and Suicide Attempts in People Prescribed Pharmaceutical Opioids for Chronic Pain. Clin. J. Pain 2016, 32, 292–301. [Google Scholar] [CrossRef]
- Brown, L.A.; Lynch, K.G.; Cheatle, M. Pain catastrophizing as a predictor of suicidal ideation in chronic pain patients with an opiate prescription. Psychiatry Res. 2020, 286, 112893. [Google Scholar] [CrossRef]
- Racine, M. Chronic pain and suicide risk: A comprehensive review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 87 Pt B, 269–280. [Google Scholar] [CrossRef]
- Manchikanti, L.; Helm, S., II; Fellows, S.H.B.; Janata, J.W.; Pampati, V.; Grider, J.S.; Boswell, M.V. Opioid epidemic in the United States. Pain Physician 2012, 15 (Suppl. 3), Es9–Es38. [Google Scholar] [CrossRef]
- Bohnert, A.S.B.; Ilgen, M.A. Understanding Links among Opioid Use, Overdose, and Suicide. N. Engl. J. Med. 2019, 380, 71–79. [Google Scholar] [CrossRef]
- Bohnert, A.S.B.; Ilgen, M.A.; Ignacio, R.V.; McCarthy, J.F.; Valenstein, M.; Blow, F.C. Risk of Death From Accidental Overdose Associated With Psychiatric and Substance Use Disorders. Am. J. Psychiatry 2012, 169, 64–70. [Google Scholar] [CrossRef]
- Bohnert, K.M.; Ilgen, M.A.; Louzon, S.; McCarthy, J.F.; Katz, I.R. Substance use disorders and the risk of suicide mortality among men and women in the US Veterans Health Administration. Addiction 2017, 112, 1193–1201. [Google Scholar] [CrossRef]
- Elman, I.; Borsook, D.; Volkow, N.D. Pain and suicidality: Insights from reward and addiction neuroscience. Prog. Neurobiol. 2013, 109, 1–27. [Google Scholar] [CrossRef]
- Espinet, S.; Corrin, T.; Baliunas, D.; Quilty, L.; Zawertailo, L.; Rizvi, S.J.; De Ruiter, W.; Bonato, S.; De Luca, V.; Kennedy, S.; et al. Predisposing and protective factors influencing suicide ideation, attempt, and death in patients accessing substance use treatment: A systematic review and meta-analysis protocol. Syst. Rev. 2019, 8, 115. [Google Scholar] [CrossRef]
- McCarthy, J.F.; Cooper, S.A.; Dent, K.R.; Eagan, A.E.; Matarazzo, B.B.; Hannemann, C.M.; Reger, M.A.; Landes, S.J.; Trafton, J.A.; Schoenbaum, M.; et al. Evaluation of the Recovery Engagement and Coordination for Health-Veterans Enhanced Treatment Suicide Risk Modeling Clinical Program in the Veterans Health Administration. JAMA Netw. Open 2021, 4, e2129900. [Google Scholar] [CrossRef]
- Belsher, B.E.; Smolenski, D.J.; Pruitt, L.D.; Bush, N.E.; Beech, E.H.; Workman, D.E.; Morgan, R.L.; Evatt, D.P.; Tucker, J.; Skopp, N.A. Prediction Models for Suicide Attempts and Deaths: A Systematic Review and Simulation. JAMA Psychiatry 2019, 76, 642–651. [Google Scholar] [CrossRef]
- Lopez-Morinigo, J.-D.; Fernandes, A.C.; Shetty, H.; Ayesa-Arriola, R.; Bari, A.; Stewart, R.; Dutta, R. Can risk assessment predict suicide in secondary mental healthcare? Findings from the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) Case Register. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1161–1171. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Song, W.; Jiang, S.; Shen, C.; Wang, X. ROC analysis of three-dimensional psychological pain in suicide ideation and suicide attempt among patients with major depressive disorder. J. Clin. Psychol. 2020, 76, 210–227. [Google Scholar] [CrossRef]
- Bryant, R.A.; O’Donnell, M.L.; Forbes, D.; McFarlane, A.C.; Silove, D.; Creamer, M. The course of suicide risk following traumatic injury. J. Clin. Psychiatry 2016, 77, 648–653. [Google Scholar] [CrossRef]
- Campbell, G.; Darke, S.; Degenhardt, L.; Townsend, H.; Carter, G.; Draper, B.; Farrell, M.; Duflou, J.; Lappin, J. Prevalence and Characteristics Associated with Chronic Noncancer Pain in Suicide Decedents: A National Study. Suicide Life Threat. Behav. 2020, 50, 778–791. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022): Cochrane. 2021. Available online: www.training.cochrane.org/handbook (accessed on 1 August 2022).
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Merskey, H.E. Classification of Chronic Pain. In Classification of Chronic Pain, 2nd ed.; IASP Press: Washington, DC, USA, 1994; pp. 209–214. [Google Scholar]
- Tossani, E. The Concept of Mental Pain. Psychother. Psychosom. 2013, 82, 67–73. [Google Scholar] [CrossRef]
- Grunebaum, M.F.; Galfalvy, H.C.; Choo, T.H.; Keilp, J.G.; Moitra, V.K.; Parris, M.S.; Marver, J.E.; Burke, A.K.; Milak, M.S.; Sublette, M.E.; et al. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am. J. Psychiatry 2018, 175, 327–335. [Google Scholar] [CrossRef]
- Miller, I.W.; Camargo, C.A., Jr.; Arias, S.A.; Sullivan, A.F.; Allen, M.H.; Goldstein, A.B.; Manton, A.P.; Espinola, J.A.; Jones, R.; Hasegawa, k.; et al. Suicide Prevention in an Emergency Department Population: The ED-SAFE Study. JAMA Psychiatry 2017, 74, 563–570. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B. Evaluating Discrimination of Risk Prediction Models: The C Statistic; Livingston, E.H., Lewis, R.J., Eds.; JAMA Guide to Statistics and Methods; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Van Calster, B.; McLernon, D.J.; van Smeden, M.; Wynants, L.; Steyerberg, E.W.; Bossuyt, P.; Bossuyt, P.; Collins, G.S.; Macaskill, P.; McLernon, D.J.; et al. Calibration: The Achilles heel of predictive analytics. BMC Med. 2019, 17, 230. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software 2021. Available online: https://www.covidence.org/ (accessed on 1 August 2022).
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Klonsky, E.D.; May, A.M.; Saffer, B.Y. Suicide, Suicide Attempts, and Suicidal Ideation. Annu. Rev. Clin. Psychol. 2016, 12, 307–330. [Google Scholar] [CrossRef]
- O’Connor, E.; Gaynes, B.; Burda, B.U.; Williams, C.; Whitlock, E.P. Screening for Suicide Risk in Primary Care: A Systematic Evidence Review for the U.S. Preventive Services Task Force; Evidence Synthesis No. 103. AHRQ Publication No. 13-05188-EF-1; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013.
- Saito, T.; Rehmsmeier, M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE 2015, 10, e0118432. [Google Scholar] [CrossRef]
- MacKinnon, D.R.; Farberow, N.L. An assessment of the utility of suicide prediction. Suicide Life Threat. Behav. 1976, 6, 86–91. [Google Scholar]
- Akechi, T.; Okamura, H.; Nishiwaki, Y.; Uchitomi, Y. Predictive factors for suicidal ideation in patients with unresectable lung carcinoma: A 6-month follow-up study. Cancer 2002, 95, 1085–1093. [Google Scholar] [CrossRef]
- Akechi, T.; Okuyama, T.; Uchida, M.; Kubota, Y.; Hasegawa, T.; Suzuki, N.; Komatsu, H.; Kusumoto, S.; Iida, S. Factors associated with suicidal ideation in patients with multiple myeloma. Jpn. J. Clin. Oncol. 2020, 50, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Phillips, K.M.; Miller, S.N. Suicidal Ideation among Veterans Living with Cancer Referred to Mental Health. Clin. Gerontol. 2020, 43, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Draper, B.; Harvey, L.; Brodaty, H.; Close, J. The association of physical illness and self-harm resulting in hospitalisation among older people in a population-based study. Aging Ment. Health 2017, 21, 279–288. [Google Scholar] [CrossRef]
- Ozdemiroglu, F.; Memis, C.O.; Meydan, N.; Dogan, B.; Kilic, S.M.; Sevincok, L.; Karakus, K. Self-Esteem, Pain and Suicidal Thoughts in a Sample of Cancer Patients. J. Mood Disord. 2017, 7, 156–162. [Google Scholar] [CrossRef]
- Park, S.A.; Chung, S.H.; Lee, Y. Factors associated with suicide risk in advanced cancer patients: A cross-sectional study. Asian Pac. J. Cancer Prev. 2016, 17, 4831–4836. [Google Scholar]
- Walker, J.; Waters, R.A.; Murray, G.; Swanson, H.; Hibberd, C.J.; Rush, R.W.; Storey, D.J.; Strong, V.A.; Fallon, M.T.; Wall, L.R.; et al. Better off dead: Suicidal thoughts in cancer patients. J. Clin. Oncol. 2008, 26, 4725–4730. [Google Scholar] [CrossRef]
- Webb, R.T.; Kontopantelis, E.; Doran, T.; Qin, P.; Creed, F.; Kapur, N. Risk of self-harm in physically ill patients in UK primary care. J. Psychosom. Res. 2012, 73, 92–97. [Google Scholar] [CrossRef]
- Xu, Q.; Jia, S.; Fukasawa, M.; Lin, L.; Na, J.; Mu, Z.; Li, B.; Li, N.; Zhao, T.; Ju, Z.; et al. A cross-sectional study on associations of physical symptoms, health self-efficacy, and suicidal ideation among Chinese hospitalized cancer patients. BMC Psychiatry 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Zhang, Z.; Sun, H.; Garg, S.; Yang, Y.; Wang, H. Suicidal Ideation in Newly-Diagnosed Chinese Cancer Patients. Front. Psychiatry 2020, 11, 708. [Google Scholar] [CrossRef]
- Zhong, B.L.; Li, S.H.; Lv, S.Y.; Tian, S.L.; Liu, Z.D.; Li, X.B.; Zhuang, H.-Q.; Tao, R.; Zhang, W.; Zhuo, C.-J. Suicidal ideation among Chinese cancer inpatients of general hospitals: Prevalence and correlates. Oncotarget 2017, 8, 25141–25150. [Google Scholar] [CrossRef]
- Alacreu-Crespo, A.; Cazals, A.; Courtet, P.; Olié, E. Brief assessment of psychological pain to predict suicidal events at one year in depressed patients. Psychother. Psychosom. 2020, 89, 320–323. [Google Scholar] [CrossRef]
- Gensichen, J.; Teising, A.; Konig, J.; Gerlach, F.M.; Petersen, J.J. Predictors of suicidal ideation in depressive primary care patients. J. Affect. Disord. 2010, 125, 124–127. [Google Scholar] [CrossRef]
- Li, H.; Xie, W.; Luo, X.; Fu, R.; Shi, C.; Ying, X.; Wang, N.; Yin, Q.; Wang, X. Clarifying the role of psychological pain in the risks of suicidal ideation and suicidal acts among patients with major depressive episodes. Suicide Life-Threat. Behav. 2014, 44, 78–88. [Google Scholar] [CrossRef]
- Caceda, R.; Durand, D.; Cortes, E.; Prendes-Alvarez, S.; Moskovciak, T.; Harvey, P.D.; Nemeroff, C.B. Impulsive Choice and Psychological Pain in Acutely Suicidal Depressed Patients. Psychosom Med. 2014, 76, 445–451. [Google Scholar] [CrossRef]
- Fishbain, D.A.; Bruns, D.; Lewis, J.E.; Disorbio, J.M.; Gao, J.; Meyer, L.J. Predictors of Homicide-Suicide Affirmation in Acute and Chronic Pain Patients. Pain Med. 2011, 12, 127–137. [Google Scholar] [CrossRef]
- Fishbain, D.A.; Bruns, D.; Meyer, L.J.; Lewis, J.E.; Gao, J.; Disorbio, J.M. Exploration of the relationship between disability perception, preference for death over disability, and suicidality in patients with acute and chronic pain. Pain Med. 2012, 13, 552–561. [Google Scholar] [CrossRef]
- Fishbain, D.A.; Lewis, J.E.; Bruns, D.; Gao, J.; Disorbio, J.M.; Meyer, L. Patient predictor variables for six forms of suicidality. Eur. J. Pain 2012, 16, 706–717. [Google Scholar] [CrossRef]
- Fishbain, D.A.; Lewis, J.E.; Gao, J.; Cole, B.; Steele Rosomoff, R. Are chronic low back pain patients who smoke at greater risk for suicide ideation? Pain Med. 2009, 10, 340–346. [Google Scholar] [CrossRef]
- McKernan, L.C.; Lenert, M.C.; Crofford, L.J.; Walsh, C.G. Outpatient Engagement and Predicted Risk of Suicide Attempts in Fibromyalgia. Arthritis Care Res. 2019, 71, 1255–1263. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Dasgupta, A.; Ward, M.M. Suicidal ideation among adults with arthritis: Prevalence and subgroups at highest risk. Data from the 2007-2008 National Health and Nutrition Examination Survey. Arthritis Care Res. 2011, 63, 1322–1333. [Google Scholar] [CrossRef]
- Tang, N.K.; Crane, C. Suicidality in chronic pain: A review of the prevalence, risk factors and psychological links. Psychol. Med. 2006, 36, 575–586. [Google Scholar] [CrossRef]
- Iams, J.D.; Newman, R.B.; Thom, E.A.; Goldenberg, R.L.; Mueller-Heubach, E.; Moawad, A.; Sibai, B.M.; Caritis, S.N.; Miodovnik, M.; Paul, R.H.; et al. Frequency of Uterine Contractions and the Risk of Spontaneous Preterm Delivery. N. Engl. J. Med. 2002, 346, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J. Managing Unstructured Big Data in Healthcare System. Healthc Inf. Res. 2019, 25, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kreimeyer, K.; Foster, M.; Pandey, A.; Arya, N.; Halford, G.; Jones, S.F.; Forshee, R.; Walderhaug, M.; Botsis, T. Natural language processing systems for capturing and standardizing unstructured clinical information: A systematic review. J. Biomed. Inform. 2017, 73, 14–29. [Google Scholar] [CrossRef]
- Obeid, J.S.; Dahne, J.; Christensen, S.; Howard, S.; Crawford, T.; Frey, L.J.; Stecker, T.; Bunnell, B.E. Identifying and Predicting Intentional Self-Harm in Electronic Health Record Clinical Notes: Deep Learning Approach. JMIR Med. Inform. 2020, 8, e17784. [Google Scholar] [CrossRef]
- Romero-Brufau, S.; Huddleston, J.M.; Escobar, G.J.; Liebow, M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit. Care 2015, 19, 285. [Google Scholar] [CrossRef]
- Lo-Ciganic, W.-H.; Huang, J.L.; Zhang, H.H.; Weiss, J.C.; Wu, Y.; Kwoh, C.K.; Donohue, J.M.; Cochran, G.; Gordon, A.J.; Malone, D.C.; et al. Evaluation of Machine-Learning Algorithms for Predicting Opioid Overdose Risk Among Medicare Beneficiaries With Opioid Prescriptions. JAMA Netw. Open 2019, 2, e190968. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Mans, C. 11—Research Study Design; Miller, R.E., Lamberski, N., Calle, P.P., Eds.; Fowler’s Zoo and Wild Animal Medicine Current Therapy; W.B. Saunders: Philadelphia, PA, USA, 2019; Volume 9, pp. 59–62. [Google Scholar]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
| Author, Year | Country | Study Design | Type of Data Sources | Study Population a | Total # Pts | Outcome (s) | Statistical Methods | Validation | C-Statistic | Accuracy | Sensitivity | PPV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fishbain, 2009 | USA | Cross-sectional | Single site questionnaire | Chronic low back pain pts who smoke | 81 | SI | Logistic regression | No validation | N/A | 0.78 | N/A | N/A |
| Fishbain, 2011 | USA | Cross-sectional | Community questionnaire (multisite) | Rehabilitation pain pts | 2264 | SI | Logistic regression | No validation | N/A | 0.96 | N/A | N/A |
| Fishbain, 2012 | USA | Cross-sectional | Community questionnaire (multisite) | Rehabilitation pain pts | 2264 | SI b | Logistic regression | No validation | N/A | 0.78–0.88 | N/A | N/A |
| Fishbain, 2012 | USA | Cross-sectional | Community questionnaire (multisite) | Rehabilitation pain pts | 2264 | SB | Logistic regression | No validation | N/A | 0.87–0.95 | N/A | N/A |
| Lopez-Morinigo, 2018 | UK | Retrospective cohort | Single site EMR | Pts seen in a comprehensive pain clinic | 13,758 | SD | Cox proportional hazards model | No validation | 0.67 | N/A | 0.65 | 0.01 |
| McKernan, 2018 | USA | Case-control | Single site EMR | Pts with fibromyalgia | 8879 | SI & SA | Bootstrapped L-1 penalized regression | Independent sample to test the external validation of published SPMs | 0.82 (SA), 0.80 (SI) | N/A | N/A | 0.08 (SA), 0.14 (SI) |
| Sun, 2020 | China | Cross-sectional | Single site chart review, Single site questionnaire | Psychiatric outpatients with major depressive disorder | 137 | Past SI & SA | Logistic regression | No validation | 0.84 | N/A | 0.91 | 0.43 |
| Tektonidou, 2011 | USA | Cross-sectional | Nationwide questionnaire | Pts aged ≥40 with arthritis, diabetes, or cancer | 2344 | SI | Random forest model | Bootstrap, Cross-validation | N/A | 1 c | N/A | N/A |
| Risk Factors | Number of Studies | % of the 87 Studies | Data Source that can be Used to Identify Risk Factors b |
|---|---|---|---|
| Depression/depressive disorders and their severity | 29 | 33% | Structured/Unstructured/Collected data c |
| Any unspecified physical or somatic pain conditions | 17 | 19% | Structured |
| Anxiety disorders and their severity | 12 | 14% | Structured/Unstructured/Collected data |
| History of suicidal behavior/ideation/attempts/suicidality | 8 | 9% | Structured/Unstructured/Collected data |
| Pain duration/severity/intensity | 8 | 9% | Unstructured/Collected data |
| Sleep disorders including insomnia | 8 | 9% | Structured |
| Age | 7 | 8% | Structured |
| Psychache/mental pain | 7 | 8% | Unstructured/Collected data |
| PTSD | 6 | 7% | Structured |
| Fibromyalgia pain | 5 | 6% | Structured |
| Gender | 5 | 6% | Structured |
| Migraine/headaches and frequency | 5 | 6% | Structured |
| Opioid use and dosage (e.g., >100 MME) | 5 | 6% | Structured |
| Perceived burdensomeness | 5 | 5% | Unstructured/Collected data |
| Antidepressant use and type | 4 | 5% | Structured |
| Comorbidity or comorbidity index | 4 | 5% | Structured |
| Perceived/feeling hopeless | 4 | 5% | Unstructured/Collected data |
| Race/ethnicity | 4 | 5% | Structured |
| AUD | 3 | 3% | Structured |
| Anger issues | 3 | 3% | Structured/Unstructured/Collected data |
| Any mental health illness | 3 | 3% | Structured |
| Any unspecified physical health illness | 3 | 3% | Structured |
| Back pain/low back pain | 3 | 3% | Structured |
| Cancer pain | 3 | 3% | Structured/Unstructured/Collected data |
| Drug use disorders | 3 | 3% | Structured |
| History of sexual/physical abuse | 3 | 3% | Structured/Unstructured/Collected data |
| Marital status (e.g., unmarried) | 3 | 3% | Structured/Unstructured/Collected data |
| Mental quality of life | 3 | 3% | Unstructured/Collected data |
| Pain catastrophizing | 3 | 3% | Unstructured/Collected data |
| Perceived/feeling stressful | 3 | 3% | Unstructured/Collected data |
| Respiratory diseases | 3 | 3% | Structured |
| Unemployment | 3 | 3% | Unstructured/Collected data |
| Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting | |
|---|---|---|---|---|---|---|
| Fishbain, 2009 | Moderate | Moderate | Low | High | Moderate | Moderate |
| Fishbain, 2011 | High | Moderate | Low | Moderate | Moderate | Moderate |
| Fishbain, 2012 | High | Moderate | Low | Moderate | Moderate | Moderate |
| Fishbain, 2012 | Low | Low | Low | Low | Low | Low |
| Lopez-Morinigo, 2018 | Low | Low | Low | Low | Low | Low |
| McKernan, 2018 | Low | Low | Low | Low | Low | Low |
| Sun, 2020 | Low | Low | Moderate | Low | Low | Low |
| Tektonidou, 2011 | Low | Low | Low | Low | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Lewis, M.O.; Bao, Y.; Adekkanattu, P.; Adkins, L.E.; Banerjee, S.; Bian, J.; Gellad, W.F.; Goodin, A.J.; Luo, Y.; et al. Predictive Modeling for Suicide-Related Outcomes and Risk Factors among Patients with Pain Conditions: A Systematic Review. J. Clin. Med. 2022, 11, 4813. https://doi.org/10.3390/jcm11164813
Huang S, Lewis MO, Bao Y, Adekkanattu P, Adkins LE, Banerjee S, Bian J, Gellad WF, Goodin AJ, Luo Y, et al. Predictive Modeling for Suicide-Related Outcomes and Risk Factors among Patients with Pain Conditions: A Systematic Review. Journal of Clinical Medicine. 2022; 11(16):4813. https://doi.org/10.3390/jcm11164813
Chicago/Turabian StyleHuang, Shu, Motomori O. Lewis, Yuhua Bao, Prakash Adekkanattu, Lauren E. Adkins, Samprit Banerjee, Jiang Bian, Walid F. Gellad, Amie J. Goodin, Yuan Luo, and et al. 2022. "Predictive Modeling for Suicide-Related Outcomes and Risk Factors among Patients with Pain Conditions: A Systematic Review" Journal of Clinical Medicine 11, no. 16: 4813. https://doi.org/10.3390/jcm11164813







