Expression of ABCA1 Transporter and LXRA/LXRB Receptors in Placenta of Women with Late Onset Preeclampsia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. RNA Extraction and cDNA Synthesis
2.3. Real-Time qPCR
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Placental Expression of LXRA, LXRB and ABCA1
3.3. Proteins Concentration in the Placentas
3.4. Correlation between Placental Protein Concentration and mRNA Expression Levels
3.5. Association between Placental mRNA and Protein Expression and Late-Onset Preeclamsia
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeyabalan, A. Epidemiology of preeclampsia: Impact of obesity. Nutr. Rev. 2013, 71, S18–S25. [Google Scholar] [CrossRef]
- Vousden, N.; Lawley, E.; Seed, P.T.; Gidiri, M.F.; Goudar, S.; Sandall, J.; Chappell, L.C.; Shennan, A.H.; on behalf of the CRADLE Trial Collaborative Group. Incidence of eclampsia and related complications across 10 low- and middle-resource geographical regions: Secondary analysis of a cluster randomised controlled trial. PLoS Med. 2019, 16, e1002775. [Google Scholar] [CrossRef] [PubMed]
- Trends in Maternal Mortality: 1990 to 2015: Estimates by WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. World Health Organization. 2015. Available online: http://apps.who.int/iris/bitstream/10665/194254/1/9789241565141_eng.pdf?ua=1 (accessed on 26 April 2021).
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2013, 3, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Benton, S.J.; von Dadelszen, P.; Roberts, J.M.; Taylor, R.N.; Powers, R.W.; Charnock-Jones, D.S.; Redman, C.W. Redefining Preeclampsia Using Placenta-Derived Biomarkers. Hypertension 2013, 61, 932–942. [Google Scholar] [CrossRef]
- Vatten, L.J.; Skjaerven, R. Is pre-eclampsia more than one disease? BJOG—Int. J. Obstet. Gynaecol. 2004, 111, 298–302. [Google Scholar] [CrossRef] [PubMed]
- ICD-10-CM Codes: Pre-Eclampsia, 2018/19. Available online: https://www.icd10data.com/ICD10CM/Codes/O00-O9A/O10-O16/O14 (accessed on 26 April 2020).
- Cornelius, D.C. Preeclampsia: From Inflammation to Immunoregulation. Clin. Med. Insights Blood Disord. 2018, 11, 1179545X17752325. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Blachnio-Zabielska, A.; Raba, G.; Sakowicz, A.; Kalinka, J.; Chabowski, A.; Laudanski, P. Sphingolipids as a new factor in the pathomechanism of preeclampsia–Mass spectrometry analysis. PLoS ONE 2017, 12, e0177601. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Gao, Y.; Gao, Y.; Liang, G.; Chen, Q.; Jiang, S.; Yang, X.; Fan, C.; Wang, H.; Wang, J.; et al. Distinct placental molecular processes associated with early-onset and late-onset preeclampsia. Theranostics 2021, 11, 5028–5044. [Google Scholar] [CrossRef]
- Liberis, A.; Stanulov, G.; Ali, E.C.; Hassan, A.; Pagalos, A.; Kontomanolis, E. Pre-eclampsia and the vascular endothelial growth factor: A new aspect. Clin. Exp. Obstet. Gynecol. 2016, 43, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Mikołajczak, P.; Kujawski, R.; Wielgus, K.; Klejewski, A.; Wolski, H.; Seremak-Mrozikiewicz, A. Pharmacological effect of quercetin in hypertension and its potential application in pregnancy-induced hypertension: Review of in vitro, in vivo, and clinical studies. Evid.-Based Complement. Alternat. Med. 2018, 2018, 7421489. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Escudero, C. The placenta in preeclampsia. Pregnancy Hypertens. 2012, 2, 72–83. [Google Scholar] [CrossRef]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, J.; Rossouw, T.M.; Lombaard, H.A.; Ehlers, M.M.; Kock, M.M. Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front. Immunol. 2018, 9, 1659. [Google Scholar] [CrossRef] [PubMed]
- Dimmick, J.E.; Kalousek, D.K. Developmental Pathology of the Embryo and Fetus; J.B. Lippincott Williams & Wilkins: New York, NY, USA, 1992; pp. 1–25. [Google Scholar]
- Plösch, T.; van Straten, E.; Kuipers, F. Cholesterol Transport by the Placenta: Placental Liver X Receptor Activity as a Modulator of Fetal Cholesterol Metabolism? Placenta 2007, 28, 604–610. [Google Scholar] [CrossRef]
- Spracklen, C.N.; Smith, C.J.; Saftlas, A.F.; Robinson, J.G.; Ryckman, K.K. Maternal Hyperlipidemia and the Risk of Preeclampsia: A Meta-Analysis. Am. J. Epidemiol. 2014, 180, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Woollett, L. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32, S218–S221. [Google Scholar] [CrossRef]
- Fuenzalida, B.; Cantin, C.; Sampada, K.; Carvajal, L.; Pastén, V.; Contreras-Duarte, S.; Albrecht, C.; Gutierrez, J.; Leiva, A. Cholesterol uptake and efflux are impaired in human trophoblast cells from pregnancies with maternal supraphysiologicalhypercholesterolemia. Sci. Rep. 2020, 10, 5264. [Google Scholar] [CrossRef]
- Fuenzalida, B.; Sobrevia, B.; Cantin, C.; Carvajal, L.; Salsoso, R.; Gutiérrez, J.; Contreras-Duarte, S.; Sobrevia, L.; Leiva, A. Maternal supraphysiological hypercholesterolemia associates with endothelial dysfunction of the placental microvasculature. Sci. Rep. 2018, 8, 7690. [Google Scholar] [CrossRef]
- Islam, S.; Choudhury, K.N.; Mainuddin, A.; Wahiduzzaman, M. Serum lipid profile and its association with hypertension in Bangladesh. Vasc. Health Risk Manag. 2014, 10, 327–332. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, D.; Chen, L. Lipid profile and cytokines in hypertension of pregnancy: A comparison of preeclampsia therapies. J. Clin. Hypertens. 2018, 20, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Kallol, S.; Huang, X.; Müller, S.; Ontsouka, C.E.; Albrecht, C. Novel Insights into Concepts and Directionality of Maternal–Fetal Cholesterol Transfer across the Human Placenta. Int. J. Mol. Sci. 2018, 19, 2334. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.A.; Vaidya, S.S.; St-Pierre, M.V.; Mikheev, A.M.; Desino, K.E.; Nyandege, A.N.; Audus, K.L.; Unadkat, J.D.; Gerk, P.M. Placental ABC Transporters: Biological Impact and Pharmaceutical Significance. Pharm. Res. 2016, 33, 2847–2878. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Anderle, P.; Hostettler, L.; Baumann, M.U.; Surbek, D.V.; Ontsouka, E.C.; Albrecht, C. Identification of placental nutrient transporters associated with intrauterine growth restriction and pre-eclampsia. BMC Genom. 2018, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.; Collins, H.L.; Jin, W.; Zanotti, I.; Favari, E.; Rothblat, G.H. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Demina, E.P.; Miroshnikova, V.V.; Schwarzman, A.L. Role of the ABC transporters A1 and G1, key reverse cholesterol transport proteins, in atherosclerosis. Mol. Biol. 2016, 50, 193–199. [Google Scholar] [CrossRef]
- Phillips, M.C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 2018, 59, 749–763. [Google Scholar] [CrossRef]
- Ye, D.; Lammers, B.; Zhao, Y.; Meurs, I.; Van Berkel, T.J.; Van Eck. M. ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: Important targets for the treatment of atherosclerosclerosis. Curr. Drug Targets 2011, 12, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Körner, M.; Wenger, F.; Nikitina, L.; Baumann, M.; Surbek, D.; Albrecht, C. PP141. The lipid transporters ABCA1 and ABCG1 are differentially expressed in preeclamptic and IUGR placentas. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2012, 2, 315–316. [Google Scholar] [CrossRef]
- Baumann, M.; Körner, M.; Huang, X.; Wenger, F.; Surbek, D.; Albrecht, C. Placental ABCA1 and ABCG1 expression in gestational disease: Pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts. Placenta 2013, 34, 1079–1086. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, M.; Li, L.; Li, C.; Min, X.; Zheng, M. Expression and Clinical Significance of ATP-Binding Cassette Transporter 1 in Serum and Placental Tissue in Chinese Patients with Preeclampsia. Gynecol. Obstet. Investig. 2014, 78, 194–200. [Google Scholar] [CrossRef]
- Quazi, F.; Molday, R.S. Differential Phospholipid Substrates and Directional Transport by ATP-binding Cassette Proteins ABCA1, ABCA7, and ABCA4 and Disease-causing Mutants. J. Biol. Chem. 2013, 288, 34414–34426. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Soumian, S.; Tetlow, N.; Patel, P.; Sullivan, M.; Lakasing, L.; Nicolaides, K.; Williamson, C. Placental ABCA1 Expression is Reduced in Primary Antiphospholipid Syndrome Compared to Pre-eclampsia and Controls. Placenta 2007, 28, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.; Keelan, J.A. Placental ABC transporters, cellular toxicity and stress in pregnancy. Chem. Interact. 2013, 203, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Töröcsik, D.; Szanto, A.; Nagy, L. Oxysterol signaling links cholesterol metabolism and inflammation via the liver X receptor in macrophages. Mol. Asp. Med. 2009, 30, 134–152. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K. Liver X receptor in cholesterol metabolism. J. Endocrinol. 2010, 204, 233–240. [Google Scholar] [CrossRef]
- Mutemberezi, V.; Buisseret, B.; Masquelier, J.; Guillemot-Legris, O.; Alhouayek, M.; Muccioli, G.G. Oxysterol levels and metabolism in the course of neuroinflammation: Insights from in vitro and in vivo models. J. Neuroinflamm. 2018, 15, 74. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [PubMed]
- Chigusa, Y.; Kondoh, E.; Mogami, H.; Nishimura, F.; Ujita, M.; Kawasaki, K.; Fujita, K.; Tatsumi, K.; Konishi, I. ATP-Binding Cassette Transporter A1 Expression Is Decreased in Preeclamptic Placentas. Reprod. Sci. 2013, 20, 891–898. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Chatuphonprasert, W.; Jarukamjorn, K.; Ellinger, I. Physiology and Pathophysiology of Steroid Biosynthesis, Transport and Metabolism in the Human Placenta. Front. Pharmacol. 2018, 9, 1027. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R Soc. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Weedon-Fekjaer, M.S.; Dalen, K.T.; Solaas, K.; Staff, A.C.; Duttaroy, A.K.; Nebb, H.I. Activation of LXR increases acyl-CoA synthetase activity through direct regulation of ACSL3 in human placental trophoblast cells. J. Lipid Res. 2010, 51, 1886–1896. [Google Scholar] [CrossRef]
- Aye, I.L.; Waddell, B.J.; Mark, P.J.; Keelan, J.A. Placental ABCA1 and ABCG1 transporters efflux cholesterol and protect trophoblasts from oxysterol induced toxicity. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2010, 1801, 1013–1024. [Google Scholar] [CrossRef]
- Marceau, G.; Volle, D.H.; Gallot, D.; Mangelsdorf, D.J.; Sapin, V.; Lobaccaro, J.M. Placental expression of the nuclear receptors for oxysterols LXRalpha and LXRbeta during mouse and human development. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2005, 283, 175–181. [Google Scholar]
- Plösch, T.; Gellhaus, A.; van Straten, E.M.; Wolf, N.; Huijkman, N.C.; Schmidt, M.; Dunk, C.E.; Kuipers, F.; Winterhager, E. The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: A reason for alterations in preeclampsia? Placenta 2010, 31, 910–918. [Google Scholar] [CrossRef]
- Cheng-Mao, X.; Yan, L.; Li, L.; Hua, J.; Xiao-Ju, W.; Jie-Wen, Z. Placental ABCA1 Expression Is Increased in Spontaneous Preterm Deliveries Compared with Iatrogenic Preterm Deliveries and Term Deliveries. BioMed Res. Int. 2017, 2017, 8248094. [Google Scholar] [CrossRef]
- Torres-Vergara, P.; Escudero, C.; Penny, J. Drug Transport at the Brain and Endothelial Dysfunction in Preeclampsia: Implications and Perspectives. Front. Physiol. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, X.; Wu, H.-Y.; Wu, N.; Zhang, X.-J.; Wang, X.; Shang, L.-X. Relationship of Liver X Receptors α and Endoglin Levels in Serum and Placenta with Preeclampsia. PLoS ONE 2016, 11, e0163742. [Google Scholar] [CrossRef]
- Jianhua, L.; Xueqin, M.; Jifen, H. Expression and clinical significance of LXRα and SREBP-1c in placentas of preeclampsia. Open Med. 2016, 11, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Mouzat, K.; Mercier, E.; Polge, A.; Evrard, A.; Baron, S.; Balducchi, J.-P.; Brouillet, J.-P.; Lumbroso, S.; Gris, J.-C. A common polymorphism in NR1H2 (LXRbeta) is associated with preeclampsia. BMC Med. Genet. 2011, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Dunk, C.E.; Pappas, J.J.; Lye, P.; Kibschull, M.; Javam, M.; Bloise, E.; Lye, S.J.; Szyf, M.; Matthews, S.G. P-Glycoprotein (P-gp)/ABCB1 plays a functional role in extravillous trophoblast (EVT) invasion and is decreased in the pre-eclamptic placenta. J. Cell. Mol. Med. 2018, 22, 5378–5393. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| ABCA1 | GGAACAGGCTACTACCTGACCTTGG | ATCGATGGTCAGCGTGTCACTCTC |
| LXRA | GATCGAGGTGATGCTTCTGG | ACTCGAAGATGGGGTTGATG |
| LXRB | GATCGTGGACTTCGCTAAGCAAGTG | GTCCTTGCTGTAGGTGAAGTCCTTC |
| GAPDH | GAGTCAACGGATTTGGTCGTATTGG | GCCATGGGTGGAATCATATTGGAAC |
| Variables | LOPE | Controls | p |
|---|---|---|---|
| n = 16 | n = 39 | ||
| Maternal age (years) | 30.38 ± 4.50 (23–35) | 30.51 ± 3.92 (20–35) | 0.9101 |
| Gestational age (weeks) | 36.75 ± 1.77 (34–40) | 37.54 ± 1.31 (36–41) | 0.0742 |
| Systolic blood pressure (mmHg) | 175.62 ± 12.63 (160–190) | 107.18 ± 9.37 (90–120) | <0.0001 |
| Diastolic blood pressure (mmHg) | 115.62 ± 6.29 (110–130) | 66.54 ± 7.54 (50–80) | <0.0001 |
| Before pregnancy BMI (kg/m2) | 23.03 ± 4.59 (17.30–29.64) | 21.59 ± 3.38 (16.73–29.75) | 0.2024 |
| After pregnancy BMI (kg/m2) | 27.38 ± 3.43 (27.38–32.37) | 26.08 ± 3.32 (19.49–33.79) | 0.1992 |
| Caesarean section, n (%) | 16 (100.00%) | 29 (74.36%) | 0.0482 |
| Primipara, n (%) | 11 (68.75%) | 12 (30.77%) | <0.0001 * |
| Fetal male sex, n (%) | 8 (50.00%) | 20 (51.28%) | 0.9203 * |
| Infant birthweight (g) | 1921.25 ± 869.59 (910–3940) | 3472.82 ± 457.83 (2360–4440) | <0.0001 |
| 1 min Apgar score, median (IQR) | 9.00 (6.50–10) | 10.00 (10–10) | 0.0094 # |
| 5 min Apgar score, median (IQR) | 9.00 (7.75–10) | 10.00 (10–10) | 0.0075 # |
| Placenta weight (g) | 385.00 ± 173.13 (160–750) | 556.82 ± 88.14 (400–800) | 0.0014 |
| ALT (U/L), median (IQR) | 29.80 (18.60–104.70) | — | — |
| AST (U/L), median (IQR) | 42.70 (25.90–130.20) | — | — |
| Urea (mg/dL), median (IQR) | 36.25 (29.52–43.70) | — | — |
| Uremic acid (mg/dL), median (IQR) | 6.18 (5.60–7.12) | — | — |
| Total protein (g/dL), median (IQR) | 5.34 (5.14–5.76) | — | — |
| Creatinine (mg/dL), median (IQR) | 0.84 (0.70–0.88) | — | — |
| Proteinuria (mg/dL), median (IQR) | 500 (150–500) | — | — |
| Gene | LOPE n = 16 | Controls n = 39 | p |
|---|---|---|---|
| LXRA/GAPDH | 0.034 (0.008–0.106) | 0.027 (0.002–0.140) | 0.6234 |
| LXRB/GAPDH | 0.824 (0.597–1.863) | 1.468 (0.756–2.762) | 0.0625 |
| ABCA1/GAPDH | 0.983 (0.640–1.208) | 1.091 (0.789–1.485) | 0.1872 |
| Protein (ng/mL) | LOPE n = 16 | Controls n = 39 | p |
|---|---|---|---|
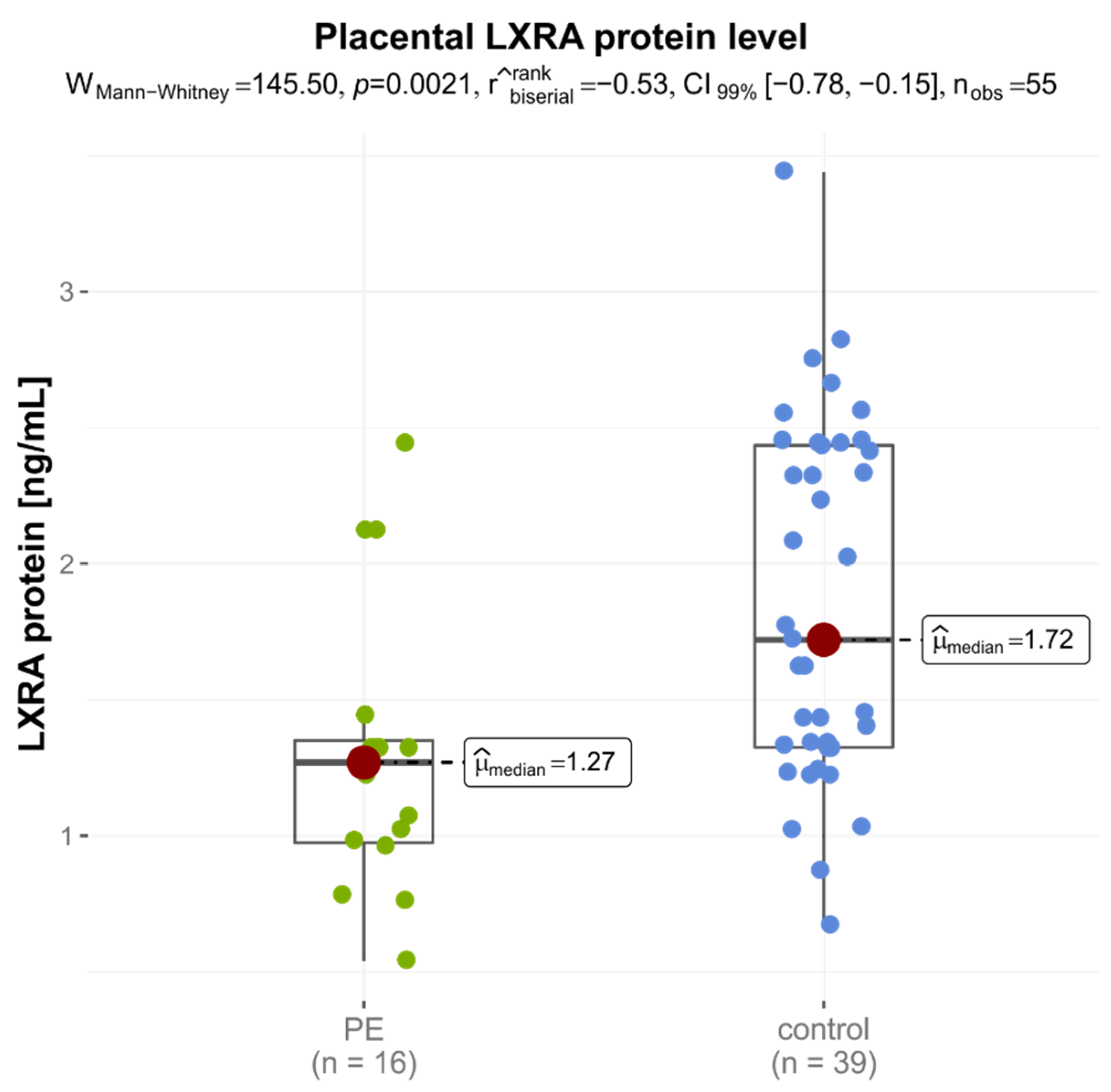
| LXRA | 1.270 (0.975–1.350) | 1.720 (1.325–2.435) | 0.0021 * |
| LXRB | 1.285 (0.785–1.570) | 1.320 (0.990–1.870) | 0.4925 |
| ABCA1 | 2.445 (1.790–2.668) | 2.220 (1.580–2.650) | 0.5043 |
| Correlations | LXRA mRNA | LXRB mRNA | ABCA1 mRNA | LXRA Protein | LXRB Protein | ABCA1 Protein |
|---|---|---|---|---|---|---|
| LXRA mRNA | 1.00 | 0.12 | −0.15 | −0.05 | 0.10 | −0.16 |
| LXRB mRNA | 0.3970 | 1.00 | 0.23 | −0.11 | 0.15 | −0.06 |
| ABCA1 mRNA | 0.2759 | 0.0844 | 1.00 | −0.08 | 0.21 | −0.10 |
| LXRA protein | 0.7296 | 0.4051 | 0.5782 | 1.00 | −0.18 | −0.20 |
| LXRB protein | 0.4599 | 0.2725 | 0.1191 | 0.1987 | 1.00 | −0.06 |
| ABCA1 protein | 0.2474 | 0.6714 | 0.4888 | 0.1478 | 0.6388 | 1.00 |
| Expression Level | ≤Median >Median | Controls n = 39 (%) | LOPE n = 16 (%) | Crude OR (95% CI) | p | AOR (95% CI) | p |
|---|---|---|---|---|---|---|---|
| LXRA mRNA | ≤0.033 | 20 (51.3) | 7 (43.8) | 1.00 | 1.00 | ||
| >0.033 | 19 (48.7) | 9 (56.2) | 1.35 (0.42–4.36) | 0.612 | 1.39 (0.38–5.09) | 0.623 | |
| LXRB mRNA | ≤1.259 | 17 (43.6) | 12 (75.0) | 1.00 | 1.00 | ||
| >1.259 | 22 (56.4) | 4 (25.0) | 0.26 (0.07–0.94) | 0.040 | 0.14 (0.02–0.82) | 0.018 | |
| ABCA1 mRNA | ≤1.058 | 18 (46.2) | 9 (56.2) | 1.00 | 1.00 | ||
| >1.058 | 21 (53.8) | 7 (43.8) | 0.67 (0.21–2.15) | 0.497 | 0.95 (0.23–3.92) | 0.945 | |
| LXRA protein | ≤1.430 | 14 (35.9) | 12 (75.0) | 1.00 | 1.00 | ||
| >1.430 | 25 (64.1) | 4 (25.0) | 0.19 (0.05–0.69) | 0.012 | 0.14 (0.03–0.63) | 0.006 | |
| LXRB protein | ≤1.320 | 17 (43.6) | 8 (50.0) | 1.00 | 1.00 | ||
| >1.320 | 22 (56.4) | 8 (50.0) | 0.77 (0.24–2.48) | 0.665 | 1.25 (0.31–4.95) | 0.755 | |
| ABCA1 protein | ≤2.320 | 20 (51.3) | 6 (37.5) | 1.00 | 1.00 | ||
| >2.320 | 19 (48.7) | 10 (62.5) | 1.75 (0.53–5.77) | 0.355 | 1.07 (0.27–4.24) | 0.922 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolski, H.; Ożarowski, M.; Kurzawińska, G.; Bogacz, A.; Wolek, M.; Łuszczyńska, M.; Drews, K.; Mrozikiewicz, A.E.; Mikołajczak, P.Ł.; Kujawski, R.; et al. Expression of ABCA1 Transporter and LXRA/LXRB Receptors in Placenta of Women with Late Onset Preeclampsia. J. Clin. Med. 2022, 11, 4809. https://doi.org/10.3390/jcm11164809
Wolski H, Ożarowski M, Kurzawińska G, Bogacz A, Wolek M, Łuszczyńska M, Drews K, Mrozikiewicz AE, Mikołajczak PŁ, Kujawski R, et al. Expression of ABCA1 Transporter and LXRA/LXRB Receptors in Placenta of Women with Late Onset Preeclampsia. Journal of Clinical Medicine. 2022; 11(16):4809. https://doi.org/10.3390/jcm11164809
Chicago/Turabian StyleWolski, Hubert, Marcin Ożarowski, Grażyna Kurzawińska, Anna Bogacz, Marlena Wolek, Małgorzata Łuszczyńska, Krzysztof Drews, Aleksandra E. Mrozikiewicz, Przemysław Ł. Mikołajczak, Radosław Kujawski, and et al. 2022. "Expression of ABCA1 Transporter and LXRA/LXRB Receptors in Placenta of Women with Late Onset Preeclampsia" Journal of Clinical Medicine 11, no. 16: 4809. https://doi.org/10.3390/jcm11164809
APA StyleWolski, H., Ożarowski, M., Kurzawińska, G., Bogacz, A., Wolek, M., Łuszczyńska, M., Drews, K., Mrozikiewicz, A. E., Mikołajczak, P. Ł., Kujawski, R., Czerny, B., Karpiński, T. M., & Seremak-Mrozikiewicz, A. (2022). Expression of ABCA1 Transporter and LXRA/LXRB Receptors in Placenta of Women with Late Onset Preeclampsia. Journal of Clinical Medicine, 11(16), 4809. https://doi.org/10.3390/jcm11164809










