Transversal Malalignment and Proximal Involvement Play a Relevant Role in Unilateral Cerebral Palsy Regardless the Subtype
Abstract
:1. Introduction
2. Patients and Methods
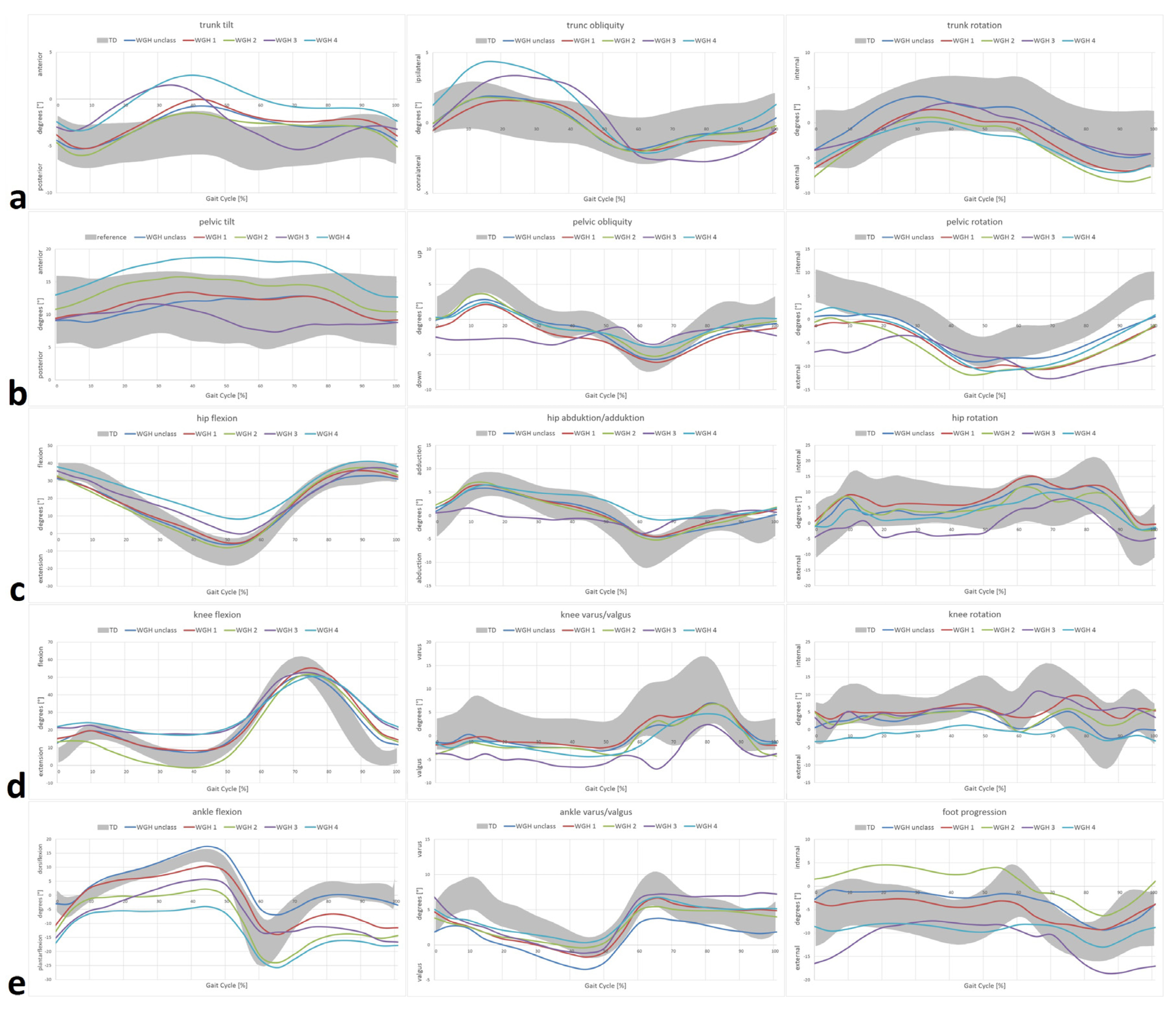
- WGH type 1: primarily drop foot in the swing phase with consecutive equinus deformity at initial contact.
- WGH type 2: equinus deformity during stance phase and swing phase with knee hyperextension during stance phase.
- WGH type 3: equinus deformity during stance phase and swing phase with restricted motion of the knee.
- WGH type 4: equinus deformity during stance phase and swing phase with restricted motion of the knee and additionally restricted motion of the hip.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colver, A.; Fairhurst, C.; Pharoah, P.O. Cerebral palsy. Lancet 2014, 383, 1240–1249. [Google Scholar] [CrossRef]
- Wren, T.A.; Rethlefsen, S.; Kay, R.M. Prevalence of specific gait abnormalities in children with cerebral palsy: Influence of cerebral palsy subtype, age, and previous surgery. J. Pediatr. Orthop. 2005, 25, 79–83. [Google Scholar] [PubMed]
- Senst, S. Unilateral Spastic Cerebral Palsy (Hemiparesis). Der. Orthopade. 2014, 43, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 15082. [Google Scholar] [CrossRef] [PubMed]
- Riad, J.; Haglund-Akerlind, Y.; Miller, F. Classification of spastic hemiplegic cerebral palsy in children. J. Pediatr. Orthop. 2007, 27, 758–764. [Google Scholar] [CrossRef] [PubMed]
- McDowell, B.C.; Kerr, C.; Kelly, C.; Salazar, J.; Cosgrove, A. The validity of an existing gait classification system when applied to a representative population of children with hemiplegia. Gait Posture 2008, 28, 442–447. [Google Scholar] [CrossRef]
- Rethlefsen, S.A.; Blumstein, G.; Kay, R.M.; Dorey, F.; Wren, T.A. Prevalence of specific gait abnormalities in children with cerebral palsy revisited: Influence of age, prior surgery, and Gross Motor Function Classification System level. Dev. Med. Child Neurol. 2017, 59, 79–88. [Google Scholar] [CrossRef]
- Domagalska, M.E.; Szopa, A.J.; Lembert, D.T. A descriptive analysis of abnormal postural patterns in children with hemiplegic cerebral palsy. Med. Sci. Monit. 2011, 17, CR110–CR116. [Google Scholar] [CrossRef]
- Kawamura, C.M.; de Morais Filho, M.C.; Barreto, M.M.; de Paula Asa, S.K.; Juliano, Y.; Novo, N.F. Comparison between visual and three-dimensional gait analysis in patients with spastic diplegic cerebral palsy. Gait Posture 2007, 25, 18–24. [Google Scholar] [CrossRef]
- Domagalska, M.; Szopa, A.; Syczewska, M.; Pietraszek, S.; Kidon, Z.; Onik, G. The relationship between clinical measurements and gait analysis data in children with cerebral palsy. Gait Posture 2013, 38, 1038–1043. [Google Scholar] [CrossRef]
- Papageorgiou, E.; Nieuwenhuys, A.; Vandekerckhove, I.; Van Campenhout, A.; Ortibus, E.; Desloovere, K. Systematic review on gait classifications in children with cerebral palsy: An update. Gait Posture 2019, 69, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Armand, S.; Decoulon, G.; Bonnefoy-Mazure, A. Gait analysis in children with cerebral palsy. EFORT Open Rev. 2016, 1, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Theologis, T.; Wright, J. Is 3-D gait analysis essential? By Professor James Wright: Introduction by Mr. Tim Theologis. Gait Posture 2015, 42, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Winters, T.F., Jr.; Gage, J.R.; Hicks, R. Gait patterns in spastic hemiplegia in children and young adults. J. Bone Joint Surg. Am. 1987, 69, 437–441. [Google Scholar] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Agostini, V.; Nascimbeni, A.; Gaffuri, A.; Knaflitz, M. Multiple gait patterns within the same Winters class in children with hemiplegic cerebral palsy. Clin. Biomech. 2015, 30, 908–914. [Google Scholar] [CrossRef]
- Dobson, F.; Morris, M.E.; Baker, R.; Graham, H.K. Unilateral cerebral palsy: A population-based study of gait and motor function. Dev. Med. Child Neurol. 2011, 53, 429–435. [Google Scholar] [CrossRef]
- Tsitlakidis, S.; Horsch, A.; Schaefer, F.; Westhauser, F.; Goetze, M.; Hagmann, S.; Klotz, M.C.M. Gait Classification in Unilateral Cerebral Palsy. J. Clin. Med. 2019, 8, 1652. [Google Scholar] [CrossRef]
- Tsitlakidis, S.; Schwarze, M.; Westhauser, F.; Heubisch, K.; Horsch, A.; Hagmann, S.; Wolf, S.I.; Gotze, M. Gait Indices for Characterization of Patients with Unilateral Cerebral Palsy. J. Clin. Med. 2020, 9, 3888. [Google Scholar] [CrossRef]
- Riad, J.; Finnbogason, T.; Broström, E. Anatomical and dynamic rotational alignment in spastic unilateral cerebral palsy. Gait Posture 2020, 81, 153–158. [Google Scholar] [CrossRef]
- Salazar-Torres, J.J.; McDowell, B.C.; Kerr, C.; Cosgrove, A.P. Pelvic kinematics and their relationship to gait type in hemiplegic cerebral palsy. Gait Posture 2011, 33, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Krzak, J.J.; Corcos, D.M.; Damiano, D.L.; Graf, A.; Hedeker, D.; Smith, P.A.; Harris, G.F. Kinematic foot types in youth with equinovarus secondary to hemiplegia. Gait Posture 2015, 41, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dobson, F.; Morris, M.E.; Baker, R.; Graham, H.K. Gait classification in children with cerebral palsy: A systematic review. Gait Posture 2007, 25, 140–152. [Google Scholar] [CrossRef]
- Tsitlakidis, S.; Beckmann, N.A.; Wolf, S.I.; Hagmann, S.; Renkawitz, T.; Götze, M. GMFCS Level-Specific Differences in Kinematics and Joint Moments of the Involved Side in Unilateral Cerebral Palsy. J. Clin. Med. 2022, 11, 2556. [Google Scholar] [CrossRef] [PubMed]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Baker, R.; Leboeuf, F.; Reay, J.; Sangeux, M. The Conventional Gait Model—Success and Limitations. In Handbook of Human Motion; Springer International Publishing: Cham, Switzerland, 2018; pp. 489–508. [Google Scholar]
- Krautwurst, B.K.; Wolf, S.I.; Heitzmann, D.W.; Gantz, S.; Braatz, F.; Dreher, T. The influence of hip abductor weakness on frontal plane motion of the trunk and pelvis in patients with cerebral palsy. Res. Dev. Disabil. 2013, 34, 1198–1203. [Google Scholar] [CrossRef]
- Gómez-Pérez, C.; Font-Llagunes, J.M.; Martori, J.C.; Vidal Samsó, J. Gait parameters in children with bilateral spastic cerebral palsy: A systematic review of randomized controlled trials. Dev. Med. Child Neurol. 2019, 61, 770–782. [Google Scholar] [CrossRef]
- Sutherland, D.H.; Davids, J.R. Common gait abnormalities of the knee in cerebral palsy. Clin. Orthop. Relat. Res. 1993, 288, 139–147. [Google Scholar]
- Rodda, J.; Graham, H.K. Classification of gait patterns in spastic hemiplegia and spastic diplegia: A basis for a management algorithm. Eur. J. Neurol. 2001, 8 (Suppl. 5), 98–108. [Google Scholar] [CrossRef]
- Szopa, A.; Domagalska-Szopa, M.; Siwiec, A.; Kwiecień-Czerwieniec, I. Canonical correlation between body-posture deviations and gait disorders in children with cerebral palsy. PLoS ONE 2020, 15, e0234654. [Google Scholar] [CrossRef]
- Kiernan, D. The relationship of trunk kinematics and kinetics with lower limb pathology during gait in children with spastic cerebral palsy. Gait Posture 2021, 86, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, D.; O’Sullivan, R.; Malone, A.; O’Brien, T.; Simms, C.K. Pathological Movements of the Pelvis and Trunk During Gait in Children with Cerebral Palsy: A Cross-Sectional Study With 3-Dimensional Kinematics and Lower Lumbar Spinal Loading. Phys. Ther. 2018, 98, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Salami, F.; Niklasch, M.; Krautwurst, B.K.; Dreher, T.; Wolf, S.I. What is the price for the Duchenne gait pattern in patients with cerebral palsy? Gait Posture 2017, 58, 453–456. [Google Scholar] [CrossRef]
- Arnold, A.S.; Delp, S.L. Rotational moment arms of the medial hamstrings and adductors vary with femoral geometry and limb position: Implications for the treatment of internally rotated gait. J. Biomech. 2001, 34, 437–447. [Google Scholar] [CrossRef]
- Arnold, A.S.; Komattu, A.V.; Delp, S.L. Internal rotation gait: A compensatory mechanism to restore abduction capacity decreased by bone deformity. Dev. Med. Child Neurol. 1997, 39, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Thielen, M.; Wolf, S.I.; Klotz, M.C.M.; Geisbüsch, A.; Putz, C.; Krautwurst, B.; Dreher, T. Supracondylar femoral rotation osteotomy affects frontal hip kinetics in children with bilateral cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 322–328. [Google Scholar] [CrossRef]
- Domagalska-Szopa, M.; Szopa, A.; Czamara, A. Dependence of Gait Deviation on Weight-Bearing Asymmetry and Postural Instability in Children with Unilateral Cerebral Palsy. PLoS ONE 2016, 11, e0165583. [Google Scholar] [CrossRef]
- Szopa, A.; Domagalska-Szopa, M.; Czamara, A. Gait pattern differences in children with unilateral cerebral palsy. Res. Dev. Disabil. 2014, 35, 2261–2266. [Google Scholar] [CrossRef]

| KINEMATICS | WGH Unclass. (n = 15) Mean [°] SD [°] | WGH Type 1 (n = 32) Mean [°] SD [°] | WGH Type 2 (n = 19) Mean [°] SD [°] | [WGH Type 3 (n = 2)] Mean [°] SD [°] | WGH Type 4 (n = 21) Mean [°] SD [°] | p-Values | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ankle flexion/extension (positive ≜ dorsiflexion; negative ≜ plantarflexion) | |||||||||||
| initial contact | −3.0 | 5.3 | −11.4 | 5.3 | −13.7 | 7.6 | −15.9 | 4.7 | −17.8 | 10.2 | p < 0.005: unclass. vs. type 1,2 and 4; p < 0.03: type 1 vs. 4 |
| stance phase maximum | 18.0 | 5.9 | 11.3 | 4.4 | 3.7 | 6.4 | 5.9 | 13.0 | 0.1 | 13.9 | p < 0.001: unclass. vs. type 2 and 4; p < 0.05: type 1 vs. type 2 and 4 |
| swing phase maximum | 2.1 | 5.3 | −5.3 | 4.5 | −11.7 | 7.4 | −10.8 | 9.0 | −14.6 | 12.3 | p < 0.005: unclass. vs. type 1, 2 and 4; p = 0.001: type 1 vs. 4 |
| ankle varus/valgus (positive ≜ varus; negative ≜ valgus) | |||||||||||
| initial contact | 1.8 | 4.1 | 4.8 | 4.1 | 3.9 | 5.4 | 7.0 | 2.5 | 5.2 | 3.7 | p > 0.05 |
| stance phase mean | −0.4 | 4.4 | 1.0 | 2.9 | 1.4 | 3.4 | 1.7 | 3.2 | 2.3 | 2.0 | p > 0.05 |
| swing phase maximum | 4.7 | 5.6 | 7.2 | 4.5 | 6.3 | 7.2 | 8.0 | 2.9 | 7.2 | 4.3 | p > 0.05 |
| foot progression (positive ≜ high; negative ≜ low) | |||||||||||
| initial contact | −3.1 | 12.8 | −3.2 | 9.1 | 1.4 | 10.8 | −16.6 | 13.2 | −8.5 | 14.3 | p > 0.05 |
| stance phase mean | −1.5 | 14.3 | −3.6 | 11.0 | 3.4 | 13.6 | −9.8 | 6.5 | −8.8 | 17.9 | p > 0.05 |
| toe off | −1.2 | 15.2 | −3.5 | 13.4 | 2.7 | 12.8 | −8.8 | 4.5 | −7.3 | 17.7 | p > 0.05 |
| swing phase mean | −6.1 | 10.9 | −7.1 | 10.7 | −2.5 | 12.4 | −14.8 | 9.7 | −10.0 | 14.2 | p > 0.05 |
| knee flexion/extension (positive ≜ flexion; negative ≜ extension) | |||||||||||
| initial contact | 12.2 | 5.5 | 14.9 | 6.9 | 13.1 | 8.0 | 21.4 | 2.6 | 21.7 | 9.8 | p < 0.05: type 4 vs. unclass., type 1 and 2 |
| stance phase minimum | 5.5 | 7.4 | 6.5 | 6.0 | −2.9 | 4.2 | 17.0 | 0.9 | 14.9 | 12.6 | p < 0.05: unclass. vs. type 2 and 4; p < 0.005: type 1 vs. 2 and 4; p < 0.001: type 2 vs. 4 |
| stance phase range | 29.3 | 6.6 | 25.7 | 7.3 | 31.5 | 5.5 | 20.3 | 1.9 | 19.8 | 8.1 | p < 0.05: type 4 vs. unclass, type 1 and 2 |
| swing phase maximum | 52.6 | 8.7 | 56.4 | 6.3 | 52.8 | 10.4 | 53.0 | 10.9 | 51.7 | 10.4 | p > 0.05 |
| knee rotation (positive ≜ internal; negative ≜ external) | |||||||||||
| initial contact | 0.4 | 9.4 | 5.4 | 7.2 | 5.3 | 7.7 | 3.9 | 9.1 | −3.4 | 9.6 | p < 0.02: type 4 vs. type 1 and 2 |
| stance phase mean | 3.3 | 8.1 | 5.2 | 5.2 | 3.8 | 6.3 | 4.5 | 9.6 | −0.9 | 8.0 | p = 0.26: type 1 vs. 4 |
| swing phase mean | 0.9 | 9.5 | 5.9 | 7.0 | 3.3 | 9.5 | 7.2 | 10.4 | −1.4 | 8.0 | p = 0.031: type 1 vs. 4 |
| knee varus/valgus (positive ≜ varus; negative ≜ valgus) | |||||||||||
| initial contact | −1.4 | 4.4 | −2.0 | 3.5 | −4.0 | 4.5 | −3.7 | 0.1 | −1.4 | 4.4 | p > 0.05 |
| stance phase mean | −1.8 | 3.9 | −1.4 | 3.2 | −2.7 | 3.8 | −5.1 | 0.7 | −2.8 | 4.1 | p > 0.05 |
| swing phase mean | 2.7 | 8.0 | 3.2 | 5.5 | 2.2 | 8.6 | −2.5 | 1.8 | 1.7 | 5.1 | p > 0.05 |
| hip flexion/extension (positive ≜ flexion; negative ≜ extension) | |||||||||||
| initial contact | 31.4 | 9.2 | 32.6 | 6.2 | 33.4 | 8.5 | 36.0 | 1.2 | 38.2 | 8.7 | p > 0.05 |
| stance phase minimum | −6.9 | 6.7 | −6.1 | 5.3 | −8.5 | 5.1 | −1.0 | 3.3 | 7.5 | 7.5 | p < 0.001: type 4 vs. unclass., type 1 and 2 |
| stance phase mean | 9.6 | 7.6 | 10.4 | 5.0 | 8.3 | 6.5 | 15.9 | 2.6 | 21.2 | 7.6 | p < 0.001: type 4 vs. unclass., type 1 and 2 |
| toe off | 0.0 | 4.8 | 0.6 | 5.2 | −0.1 | 5.4 | 4.3 | 0.6 | 11.1 | 7.1 | p < 0.001: type 4 vs. unclass., type 1 and 2 |
| swing phase maximum | 34.1 | 8.8 | 36.8 | 5.7 | 38.3 | 7.7 | 38.1 | 2.1 | 42.1 | 8.3 | p < 0.03: unclass. vs. type 4 |
| hip rotation (positive ≜ internal; negative ≜ external) | |||||||||||
| initial contact | −1.4 | 16.1 | 0.2 | 14.7 | −1.8 | 17.8 | −4.8 | 3.3 | −1.0 | 15.3 | p > 0.05 |
| stance phase minimum | −3.9 | 15.5 | −1.4 | 14.7 | −3.7 | 17.1 | −6.6 | 5.2 | −5.2 | 17.3 | p > 0.05 |
| stance phase mean | 4.6 | 14.2 | 7.0 | 14.0 | 4.7 | 15.9 | −2.1 | 3.9 | 2.6 | 16.2 | p > 0.05 |
| toe off | 11.3 | 16.9 | 14.1 | 16.1 | 11.3 | 17.6 | 4.4 | 0.5 | 6.8 | 18.6 | p > 0.05 |
| swing phase maximum | 17.2 | 16.8 | 18.4 | 14.1 | 16.3 | 14.7 | 8.1 | 0.3 | 12.9 | 16.6 | p > 0.05 |
| hip abduction/adduction (positive ≜ adduction; negative ≜ abduction) | |||||||||||
| mid stance mean | 5.0 | 4.4 | 5.4 | 4.7 | 5.7 | 4.1 | 0.2 | 3.0 | 5.8 | 7.3 | p > 0.05 |
| stance phase minimum | −4.7 | 5.7 | −4.4 | 4.4 | −4.8 | 4.7 | −4.7 | 0.5 | −2.4 | 5.4 | p > 0.05 |
| toe off | −3.9 | 7.2 | −3.7 | 4.8 | −4.4 | 5.1 | −3.8 | 1.3 | −0.1 | 5.9 | p > 0.05 |
| swing phase minimum | −6.1 | 5.5 | −5.5 | 4.5 | −6.3 | 4.0 | −4.1 | 1.6 | −3.8 | 4.5 | p > 0.05 |
| pelvic tilt (positive ≜ anterior; negative ≜ posterior) | |||||||||||
| stance phase maximum | 13.8 | 7.6 | 14.2 | 4.7 | 16.7 | 4.7 | 12.3 | 3.3 | 20.3 | 5.0 | p < 0.01: type 4 vs. unclass and type 1 |
| stance phase mean | 10.9 | 7.1 | 11.9 | 4.4 | 14.3 | 4.9 | 10.1 | 2.8 | 17.1 | 5.2 | p < 0.02: type 4 vs. unclass and type 1 |
| stance phase range | 5.7 | 1.9 | 5.7 | 2.5 | 6.3 | 1.9 | 6.3 | 2.2 | 7.8 | 3.5 | p > 0.05 |
| swing phase maximum | 13.5 | 8.6 | 13.3 | 4.3 | 15.2 | 4.5 | 10.0 | 1.5 | 19.1 | 4.9 | p < 0.04: type 4 vs. unclass and type 1 |
| swing phase mean | 11.3 | 8.1 | 11.3 | 4.2 | 12.9 | 4.6 | 8.2 | 1.0 | 16.1 | 4.5 | p = 0.02: type 1 vs. type 4 |
| swing phase range | 5.2 | 2.8 | 4.7 | 2.8 | 5.1 | 2.9 | 3.8 | 1.1 | 6.9 | 4.2 | p > 0.05 |
| pelvic rotation (positive ≜ internal; negative ≜ external) | |||||||||||
| initial contact | 0.6 | 5.6 | −1.6 | 5.5 | −0.7 | 3.9 | −7.0 | 1.9 | 1.3 | 8.4 | p > 0.05 |
| stance phase mean | −3.3 | 4.9 | −4.8 | 5.4 | −5.8 | 3.3 | −6.2 | 2.3 | −4.0 | 9.4 | p > 0.05 |
| toe off | −8.2 | 5.7 | −10.1 | 4.8 | −10.7 | 5.0 | −9.9 | 0.9 | −10.6 | 10.5 | p > 0.05 |
| swing phase mean | −4.9 | 5.4 | −7.5 | 4.8 | −7.4 | 4.2 | −10.6 | 2.0 | −6.0 | 9.4 | p > 0.05 |
| pelvic obliquity (positive ≜ up; negative ≜ down) | |||||||||||
| stance phase maximum | 3.2 | 3.0 | 2.4 | 3.2 | 4.0 | 2.7 | 0.3 | 4.0 | 3.3 | 4.0 | p = 0.010: type 1 vs. type 4 |
| stance phase mean | −0.3 | 2.8 | −1.3 | 3.2 | −0.2 | 2.6 | −2.6 | 2.5 | −0.4 | 4.3 | p > 0.05 |
| toe off | −5.3 | 3.8 | −5.5 | 4.0 | −4.8 | 3.9 | −3.1 | 5.6 | −3.6 | 3.7 | p > 0.05 |
| trunk tilt (positive ≜ anterior; negative ≜ posterior) | |||||||||||
| initial contact | −4.3 | 4.1 | −3.7 | 3.8 | −4.5 | 2.6 | −2.9 | 2.1 | −2.3 | 5.0 | p > 0.05 |
| stance phase maximum | 0.0 | 4.4 | 0.2 | 4.5 | −0.8 | 3.0 | 1.5 | 0.4 | 2.9 | 6.4 | p > 0.05 |
| stance phase range | 5.8 | 2.5 | 5.7 | 2.2 | 5.5 | 2.7 | 5.6 | 2.5 | 6.9 | 2.5 | p > 0.05 |
| swing phase maximum | −1.2 | 4.7 | −1.3 | 4.1 | −1.9 | 3.0 | −2.6 | 2.5 | 1.1 | 5.5 | p > 0.05 |
| swing phase range | 3.8 | 2.7 | 3.0 | 1.6 | 3.4 | 2.4 | 3.0 | 1.4 | 4.2 | 2.6 | p > 0.05 |
| trunk rotation (positive ≜ internal; negative ≜ external) | |||||||||||
| initial contact | −4.0 | 5.2 | −6.6 | 5.7 | −7.9 | 5.9 | −3.9 | 1.8 | −6.0 | 9.8 | p > 0.05 |
| mid stance mean | 1.8 | 5.8 | −0.9 | 5.5 | −1.3 | 6.0 | −0.9 | 4.5 | −1.5 | 7.2 | p > 0.05 |
| toe off | 2.1 | 6.5 | 0.0 | 5.0 | −1.0 | 5.7 | 0.6 | 3.6 | −2.0 | 8.4 | p > 0.05 |
| trunk obliquity (positive ≜ ipsilateral; negative ≜ contralateral) | |||||||||||
| initial contact | −0.1 | 2.0 | −0.6 | 1.9 | −0.1 | 2.0 | −0.4 | 0.5 | 1.1 | 2.3 | p = 0.031: type 1 vs. type 4 |
| stance phase maximum | 2.3 | 2.7 | 2.0 | 2.1 | 2.3 | 2.4 | 3.6 | 3.0 | 4.7 | 3.8 | p = 0.010: type 1 vs. type 4 |
| stance phase mean | 0.5 | 1.8 | 0.5 | 1.7 | 0.5 | 1.9 | 1.7 | 1.6 | 2.1 | 3.2 | p > 0.05 |
| swing phase maximum | 0.7 | 2.2 | −0.2 | 2.0 | 0.1 | 2.0 | −0.3 | 0.3 | 1.4 | 2.4 | p > 0.05 |
| swing phase mean | −0.9 | 2.1 | −1.4 | 1.9 | −1.0 | 1.9 | −2.3 | 2.0 | −0.8 | 2.3 | p > 0.05 |
| Joint Moments | WGH Unclass. (n = 15) Mean [Nm/kg] SD [Nm/kg] | WGH Type 1 (n = 32) Mean [Nm/kg] SD [Nm/kg] | WGH Type 2 (n = 19) Mean [Nm/kg] SD [Nm/kg] | [WGH Type 3 (n = 2)] Mean [Nm/kg] SD [Nm/kg] | WGH Type 4 (n = 21) Mean [Nm/kg] SD [Nm/kg] | p-Values | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ankle flexion moment (positive ≜ dorsiflexion; negative ≜ plantarflexion) | |||||||||||
| initial contact | −0.026 | 0.031 | 0.026 | 0.025 | 0.042 | 0.031 | 0.025 | 0.009 | 0.020 | 0.038 | p < 0.001: unclass vs. type 1, 2 and 4 |
| stance phase minimum | −0.076 | 0.093 | 0.011 | 0.034 | 0.013 | 0.040 | 0.010 | 0.025 | −0.020 | 0.031 | p ≤ 0.02: unclass vs. type 1, 2 and 4 |
| stance phase maximum | 1.131 | 0.278 | 1.182 | 0.169 | 1.011 | 0.235 | 1.062 | 0.371 | 0.934 | 0.346 | p = 0.007: type 1 vs. 4 |
| stance phase mean | 0.552 | 0.136 | 0.715 | 0.117 | 0.664 | 0.142 | 0.646 | 0.166 | 0.558 | 0.202 | p < 0.005: type 1 vs. unclass and type 4 |
| ankle rotation moment (positive ≜ internal; negative ≜ external) | |||||||||||
| stance phase min | −0.037 | 0.034 | −0.040 | 0.030 | −0.042 | 0.040 | −0.039 | 0.023 | −0.061 | 0.039 | p > 0.005 |
| stance phase max | 0.083 | 0.054 | 0.080 | 0.035 | 0.093 | 0.047 | 0.098 | 0.049 | 0.078 | 0.057 | p > 0.005 |
| stance phase mean | 0.022 | 0.030 | 0.026 | 0.028 | 0.038 | 0.038 | 0.040 | 0.028 | 0.012 | 0.047 | p > 0.005 |
| ankle varus/valgus moment (positive ≜ varus; negative ≜ valgus) | |||||||||||
| stance phase max | 0.065 | 0.068 | 0.063 | 0.040 | 0.072 | 0.063 | 0.137 | 0.012 | 0.087 | 0.075 | p > 0.005 |
| stance phase mean | 0.004 | 0.056 | 0.007 | 0.033 | 0.024 | 0.047 | 0.079 | 0.003 | 0.023 | 0.062 | p > 0.005 |
| swing phase mean | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | p > 0.005 |
| knee flexion moment (positive ≜ flexion; negative ≜ extension) | |||||||||||
| mid stance minimum | −0.058 | 0.167 | −0.218 | 0.198 | −0.424 | 0.217 | −0.010 | 0.121 | −0.021 | 0.365 | p = 0.002: unclass vs. type 2 |
| stance phase max | 0.442 | 0.203 | 0.216 | 0.173 | 0.100 | 0.101 | 0.203 | 0.014 | 0.376 | 0.260 | p < 0.004: unclass vs. type 1 and 2; p < 0.05: type 4 vs. type 1 and 2 |
| stance phase mean | 0.063 | 0.109 | −0.070 | 0.137 | −0.208 | 0.134 | 0.067 | 0.037 | 0.094 | 0.252 | p < 0.001: unclass vs. type 2; p ≤ 0.012: type 4 vs. type 1 and 2 |
| mid swing maximum | 0.004 | 0.020 | −0.006 | 0.021 | −0.008 | 0.015 | −0.016 | 0.023 | 0.001 | 0.021 | p > 0.05 |
| knee rotation moment (positive ≜ internal; negative ≜ external) | |||||||||||
| initial contact | 0.003 | 0.005 | −0.002 | 0.007 | −0.007 | 0.009 | −0.006 | 0.006 | 0.003 | 0.008 | p ≤ 0.005: type 2 vs. unclass and type 4 |
| stance phase mean | 0.026 | 0.021 | 0.034 | 0.021 | 0.040 | 0.025 | 0.029 | 0.031 | 0.013 | 0.033 | p = 0.018: type 2 vs. type 4 |
| knee varus/valgus moment (positive ≜ varus; negative ≜ valgus) | |||||||||||
| stance phase maximum | 0.371 | 0.104 | 0.334 | 0.106 | 0.323 | 0.131 | 0.252 | 0.069 | 0.268 | 0.150 | p > 0.005 |
| stance phase mean | 0.147 | 0.070 | 0.137 | 0.075 | 0.156 | 0.095 | 0.125 | 0.061 | 0.100 | 0.127 | p > 0.005 |
| hip flexion moment (positive ≜ flexion; negative≜ extension) | |||||||||||
| initial contact | 0.218 | 0.199 | 0.354 | 0.120 | 0.325 | 0.246 | 0.300 | 0.041 | 0.290 | 0.298 | p > 0.05 |
| stance phase minimum | −0.632 | 0.174 | −0.489 | 0.175 | −0.571 | 0.174 | −0.411 | 0.083 | −0.439 | 0.196 | p = 0.021: unclass vs. type 4 |
| stance phase mean | −0.084 | 0.141 | 0.014 | 0.175 | −0.029 | 0.135 | 0.042 | 0.026 | 0.064 | 0.156 | p > 0.05 |
| swing phase mean | 0.023 | 0.025 | 0.030 | 0.018 | 0.038 | 0.026 | −0.015 | 0.015 | 0.008 | 0.036 | p ≤ 0.036: type 4 vs. type 1 and 2 |
| hip rotation moment (positive ≜ internal; negative ≜ external) | |||||||||||
| initial contact | 0.003 | 0.013 | 0.008 | 0.013 | 0.006 | 0.010 | 0.011 | 0.001 | 0.010 | 0.020 | p > 0.05 |
| mid stance maximum | 0.004 | 0.027 | 0.027 | 0.043 | 0.059 | 0.040 | −0.033 | 0.020 | −0.022 | 0.058 | p = 0.007: unclass vs. type 2; p ≤ 0.003 type 4 vs. type 1 and 2 |
| stance phase mean | −0.010 | 0.022 | 0.006 | 0.030 | 0.033 | 0.030 | −0.020 | 0.012 | −0.028 | 0.040 | p = 0.003: unclass vs. type 2; p ≤ 0.005: type 4 vs. type 1 and 2 |
| hip abduction/adduction moment (positive ≜ adduction; negative ≜ abduction) | |||||||||||
| mid stance maximum | 0.652 | 0.191 | 0.712 | 0.161 | 0.657 | 0.196 | 0.634 | 0.118 | 0.587 | 0.229 | p > 0.05 |
| stance phase max | 0.665 | 0.178 | 0.724 | 0.148 | 0.690 | 0.173 | 0.634 | 0.118 | 0.601 | 0.229 | p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitlakidis, S.; Campos, S.; Beckmann, N.A.; Wolf, S.I.; Hagmann, S.; Renkawitz, T.; Götze, M. Transversal Malalignment and Proximal Involvement Play a Relevant Role in Unilateral Cerebral Palsy Regardless the Subtype. J. Clin. Med. 2022, 11, 4816. https://doi.org/10.3390/jcm11164816
Tsitlakidis S, Campos S, Beckmann NA, Wolf SI, Hagmann S, Renkawitz T, Götze M. Transversal Malalignment and Proximal Involvement Play a Relevant Role in Unilateral Cerebral Palsy Regardless the Subtype. Journal of Clinical Medicine. 2022; 11(16):4816. https://doi.org/10.3390/jcm11164816
Chicago/Turabian StyleTsitlakidis, Stefanos, Sarah Campos, Nicholas A. Beckmann, Sebastian I. Wolf, Sébastien Hagmann, Tobias Renkawitz, and Marco Götze. 2022. "Transversal Malalignment and Proximal Involvement Play a Relevant Role in Unilateral Cerebral Palsy Regardless the Subtype" Journal of Clinical Medicine 11, no. 16: 4816. https://doi.org/10.3390/jcm11164816





