Thromboembolic Antiphospholipid Syndrome (APS): Efficacy and Safety of Different Anticoagulants-Results of the APSantiCO Registry
Abstract
:1. Introduction
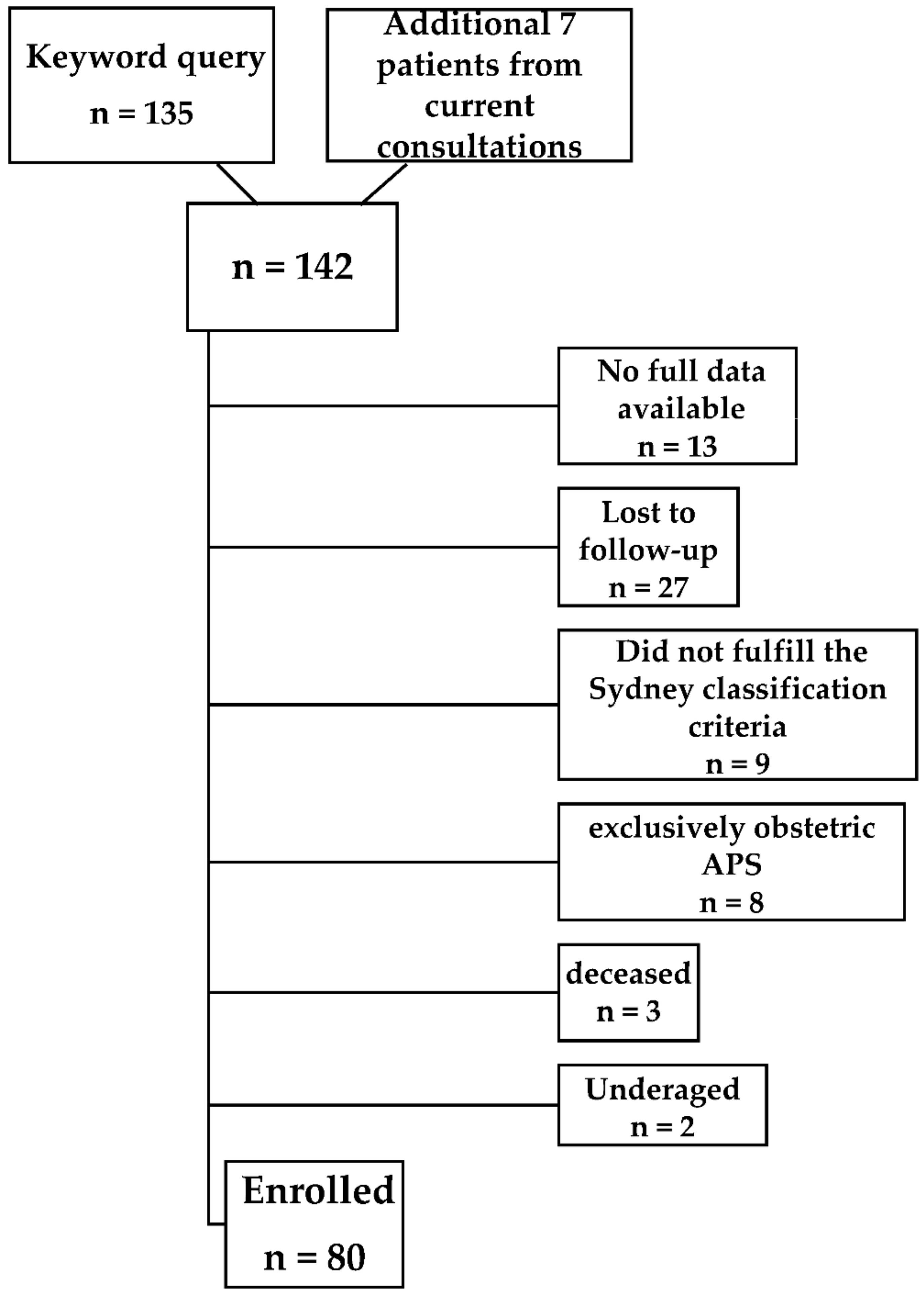
2. Materials and Methods
- -
- Minimum age of 18 years;
- -
- The presence of any APS antibody risk profile (single/double/triple positivity);
- -
- Arterial and/or venous thromboembolism.
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Recurrent Arterial Thrombosis
3.3. Recurrent Venous Thrombosis
3.4. Bleeding Events (MB and CRNMB)
4. Discussion
4.1. Recurrent Arterial and Venous Thrombosis
4.2. Bleeding Complications (MB and CRNMB)
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cervera, R.; Piette, J.-C.; Font, J.; Khamashta, M.A.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S.; Lakos, G.; Tincani, A.; Kontopoulou-Griva, I.; et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients. Arthritis Rheum. 2002, 46, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [PubMed]
- Fonseca, A.G.; D'Cruz, D.P. Controversies in the antiphospholipid syndrome: Can we ever stop warfarin? J. Autoimmune Dis. 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Cuadrado, M.J.; Erkan, D.; Duarte-Garcia, A.; Isenberg, D.A.; Knight, J.S.; Ortel, T.L.; Rahman, A.; Salmon, J.E.; Tektonidou, M.G.; et al. 16th International Congress on Antiphospholipid Antibodies Task Force Report on Antiphospholipid Syndrome Treatment Trends. Lupus 2020, 29, 1571–1593. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef]
- Cohen, H.; Efthymiou, M.; Isenberg, D.A. Use of direct oral anticoagulants in antiphospholipid syndrome. J. Thromb. Haemost. 2018, 16, 1028–1039. [Google Scholar] [CrossRef]
- Kalmanti, L.; Lindhoff-Last, E. Treatment of Vascular Thrombosis in Antiphospholipid Syndrome: An Update. Hämostaseologie 2020, 40, 031–037. [Google Scholar] [CrossRef]
- Pengo, V.; Denas, G.; Zoppellaro, G.; Jose, S.P.; Hoxha, A.; Ruffatti, A.; Andreoli, L.; Tincani, A.; Cenci, C.; Prisco, D.; et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018, 132, 1365–1371. [Google Scholar] [CrossRef]
- Pharmacovigilance Risk Assessment Committee of the European Medicines Agency. New Product Information Wording: Direct-Acting Oral Anticoagulants (DOACs): Apixaban; Dabigatran Etexilate; Edoxaban; Rivaroxaban-Recurrent Thrombosis in Patients with Antiphospholipid Syndrome (EPITT no 19320). Available online: www.ema.europa.eu/en/documents/other/new-product-information-wording-extracts-prac-recommendations-signals-adopted-8-11-april-2019-prac_en.pdf (accessed on 20 June 2022).
- Arachchillage, D.R.J.; Gomez, K.; Alikhan, R.; Anderson, J.A.M.; Lester, W.; Laffan, M. Addendum to British Society for Haematology Guidelines on Investigation and Management of Antiphospholipid syndrome: Use of direct acting oral anticoagulants. Br. J. Haematol. 2020, 189, 212–215. [Google Scholar] [CrossRef]
- Cohen, H.; Hunt, B.J.; Efthymiou, M.; Arachchillage, D.R.J.; Mackie, I.J.; Clawson, S.; Sylvestre, Y.; Machin, S.J.; Bertolaccini, M.L.; Ruiz-Castellano, M.; et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): A randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016, 3, e426–e436. [Google Scholar] [CrossRef]
- Malec, K.; Góralczyk, T.; Undas, A. The use of direct oral anticoagulants in 56 patients with antiphospholipid syndrome. Thromb. Res. 2017, 152, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Elsebaie, M.A.T.; Es, N.; Langston, A.; Büller, H.R.; Gaddh, M. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: A systematic review and meta-analysis. J. Thromb. Haemost. 2019, 17, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, R.; Langer, F.; Kalka, C.; Konstantinides, S.; Klamroth, R.; Oldenburg, J.; Schellong, S.; Scholz, U.; Stücker, M.; Lindhoff-Last, E. Treatment of the antiphospholipid syndrome with direct oral anticoagulants. Vasa 2019, 48, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Lindhoff-Last, E.; Humpich, M.; Schmitt, J.; Rödiger, S.; Seifried, E.; Bauersachs, R. MIXCON-LA: A Precise, Sensitive and Specific aPTT-Based Assay for Detection of Lupus Anticoagulant. Clin. Appl. Thromb. Hemost. 2002, 8, 163–167. [Google Scholar] [CrossRef]
- Schulman, S.; Angerås, U.; Bergqvist, D.; Eriksson, B.; Lassen, M.R.; Fisher, W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010, 8, 202–204. [Google Scholar] [CrossRef]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Liu, A.; Rupani, K.V.; Naymagon, L. Direct oral anticoagulants versus warfarin in patients with single antibody-positive anti-phospholipid syndrome. Eur. J. Haematol. 2022, 109, 69–74. [Google Scholar] [CrossRef]
- Kwan, V.; Kaplovitch, E.; Selby, R.; Abdulrehman, J. Effectiveness and safety of the direct oral anticoagulants in non-triple positive antiphospholipid syndrome without prior arterial thromboembolism. J. Thromb. Thrombolysis 2022, 53, 690–696. [Google Scholar] [CrossRef]
- Malec, K.; Broniatowska, E.; Undas, A. Direct oral anticoagulants in patients with antiphospholipid syndrome: A cohort study. Lupus 2020, 29, 37–44. [Google Scholar] [CrossRef]
- Williams, B.; Saseen, J.J.; Trujillo, T.; Palkimas, S. Direct oral anticoagulants versus warfarin in patients with single or double antibody-positive antiphospholipid syndrome. J. Thromb. Thrombolysis 2022, 54, 67–73. [Google Scholar] [CrossRef]
- Martinelli, I.; Abbattista, M.; Bucciarelli, P.; Tripodi, A.; Artoni, A.; Gianniello, F.; Novembrino, C.; Peyvandi, F. Recurrent thrombosis in patients with antiphospholipid antibodies treated with vitamin K antagonists or rivaroxaban. Haematologica 2018, 103, e315–e317. [Google Scholar] [CrossRef] [PubMed]
- Woller, S.C.; Stevens, S.M.; Kaplan, D.; Wang, T.-F.; Branch, D.W.; Groat, D.; Wilson, E.L.; Armbruster, B.; Aston, V.T.; Lloyd, J.F.; et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: A randomized trial. Blood Adv. 2022, 6, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; Marchioli, R.; Brancaccio, V.; Schinco, P.; Wisloff, F.; Musial, J.; Baudo, F.; Berrettini, M.; Testa, S.; D'Angelo, A.; et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS)1. J. Thromb. Haemost. 2005, 3, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Abdou, J.K.; Auyeung, V.; Patel, J.P.; Arya, R. Adherence to long-term anticoagulation treatment, what is known and what the future might hold. Br. J. Haematol. 2016, 174, 30–42. [Google Scholar] [CrossRef]
- Harper, P.; Pollock, D.; Stephens, M. Dabigatran persistence and adherence in New Zealand: A nationwide retrospective observational study. BMJ Open 2018, 8, e020212. [Google Scholar] [CrossRef]
- Platt, A.B.; Localio, A.R.; Brensinger, C.M.; Cruess, D.G.; Christie, J.D.; Gross, R.; Parker, C.S.; Price, M.; Metlay, J.P.; Cohen, A.; et al. Risk factors for nonadherence to warfarin: Results from the IN-RANGE study. Pharmacoepidemiol. Drug Saf. 2008, 17, 853–860. [Google Scholar] [CrossRef]
- Shore, S.; Carey, E.P.; Turakhia, M.P.; Jackevicius, C.A.; Cunningham, F.; Pilote, L.; Bradley, S.M.; Maddox, T.M.; Grunwald, G.K.; Barón, A.E.; et al. Adherence to dabigatran therapy and longitudinal patient outcomes: Insights from the Veterans Health Administration. Am. Heart J. 2014, 167, 810–817. [Google Scholar] [CrossRef]
- Beyer-Westendorf, J.; Forster, K.; Ebertz, F.; Gelbricht, V.; Schreier, T.; Gobelt, M.; Michalski, F.; Endig, H.; Sahin, K.; Tittl, L.; et al. Drug persistence with rivaroxaban therapy in atrial fibrillation patients--results from the Dresden non-interventional oral anticoagulation registry. Europace 2015, 17, 530–538. [Google Scholar] [CrossRef]
- Thorne, K.; Jayathissa, S.; Dee, S.; Briggs, N.; Taylor, J.; Reid, S.; De Silva, K.; Dean, J. Adherence and outcomes of patients prescribed dabigatran (Pradaxa) in routine clinical practice. Intern. Med. J. 2014, 44, 261–265. [Google Scholar] [CrossRef]
- Zuily, S.; Cohen, H.; Isenberg, D.; Woller, S.C.; Crowther, M.; Dufrost, V.; Wahl, D.; Doré, C.J.; Cuker, A.; Carrier, M.; et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: Guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2020, 18, 2126–2137. [Google Scholar] [CrossRef]
- Posch, F.; Gebhart, J.; Rand, J.H.; Koder, S.; Quehenberger, P.; Pengo, V.; Ay, C.; Pabinger, I. Cardiovascular risk factors are major determinants of thrombotic risk in patients with the lupus anticoagulant. BMC Med. 2017, 15, 54. [Google Scholar] [CrossRef] [PubMed]



| Study Population | Patients on VKA | Patients on DOAC | p-Value Comparing VKA vs. DOAC | Patients on Subcutaneous Anticoagulation and/or Platelet Aggregation Inhibitors | |
|---|---|---|---|---|---|
| Total number of patients | 80 | 35 | 29 * | 0.532 | 16 |
| female, % (counts/n) | 52.5% (42/80) | 51.4% (18/35) | 41.4% (12/29) | 0.460 | 75% (12/16) |
| BMI. Median (25–75% Percentiles) | 26.3 | 26.3 | 25.3 | 0.672 | 26.7 |
| (24.2–29.9) | (24.4–30.1) | (24.1–30.4) | (23.8–29.5) | ||
| Age at index thrombosis Median (years) (25–75% Percentiles) | |||||
| 44.5 | 46 | 50 | 0.504 | 35.5 | |
| (30.8–59.3) | (33–60) | (31–66) | (27–43) | ||
| Index event | |||||
| venous thromboembolism, %; (counts/n) | 71.3% (57/80) | 68.6% (24/35) | 82.8% (24/29) | 0.251 | 56.3% (9/16) |
| spontaneous venous thromboembolism | 73.7% (42/57) | 79.2% (19/24) | 58.3% (14/24) | 0.212 | 77.8% (7/9) |
| Deep leg vein thrombosis Pulmonary embolism ◊ Superficial vein thrombosis Atypical venous thrombosis | 66.7% (38/57) 26.3% (15/57) 10.5% (6/57) 15.8% (9/57) | 75% (18/24) 37.5% (9/24) 8.3% (2/24) 4.2% (1/24) | 66.7% (16/24) 20.8% (5/24) 12.5% (3/24) 20.8% (5/24) | 0.244 | 44.4% (4/9) 11.1% (1/9) 11.1% (1/9) 33.3% (3/9) |
| arterial thromboembolism, %; (counts/n) | 28.8% (23/80) | 31.4% (11/35) | 17.2% (5/29) | 0.251 | 43.8% (7/16) |
| Stroke/TIA myocardial infarction peripheral arterial occlusion atypical arterial embolism | 52.2% (12/23) 26.1% (6/23) 13% (3/23) 8.7% (2/23) | 72.7% (8/11) 18.2% (2/11) 9.1% (1/11) 0% (0/11) | 60% (3/5) 20% (1/5) 20% (1/5) 0% (0/5) | 1.000 | 57.1% (4/7) 14.3% (1/7) 14.3% (1/7) 14.3% (1/7) |
| Cardiovascular risk factors %; (counts/n) | 86.3% (69/80) | 91.4% (32/35) | 79.3% (23/29) | 0.279 | 87.5% (14/16) |
| None | 13.8% (11/80) | 8.6% (3/35) | 20.7% (6/29) | 0.279 | 12.5% (2/16) |
| Hypertension | 48.8% (39/80) | 51.4% (18/35) | 48.3% (14/29) | 1.000 | 43.8% (7/16) |
| Diabetes | 8.8% (7/80) | 8.6% (3/35) | 10.3% (3/29) | 1.000 | 6.3% (1/16) |
| Smoking | 37.5% (30/80) | 31.4% (11/35) | 34.5% (10/29) | 1.000 | 56.3% (9/16) |
| Hypercholesterolemia | 52.5% (42/80) | 68.6% (24/35) | 48.3% (14/29) | 0.128 | 25% (4/16) |
| elevated Lipoprotein a | 13.8% (11/80) | 25.7% (9/35) | 6.9% (2/29) | 0.093 | 0% (0/16) |
| atrial fibrillation %; (counts/n) | 6.3% (5/80) | 8.6% (3/35) | 3.4% (1/29) | 0.620 | 6.3% (1/16) |
| Antiphospholipid antibodies %; (counts/n) | |||||
| Lupus anticoagulants | 63.8% (51/80) | 77.1% (27/35) | 48.3% (14/29) | 0.021 | 62.5% (10/16) |
| Anticardiolipin antibodies | 80% (64/80) | 82.9% (29/35) | 72.4% (21/29) | 0.372 | 87.5% (14/16) |
| Anti-2 glycoprotein I antibodies | 70% (56/80) | 80% (28/35) | 55.2% (16/29) | 0.028 | 75% (12/16) |
| Single positivity | 27.5% (22/80) | 17.1% (6/35) | 41.4% (12/29) | 0.050 | 25% (4/16) |
| Double positivity | 31.3% (25/80) | 25.7% (9/35) | 41.4% (12/29) | 0.245 | 25% (4/16) |
| Triple positivity | 41.3% (33/80) | 57.1% (20/35) | 17.2% (5/29) | 0.002 | 50% (8/16) |
| Autoimmune disease %; (counts/n) | 27.5% (22/80) | 22.9% (8/35) | 27.6% (8/29) | 0.774 | 37.5% (6/16) |
| Inherited thrombophilia %; (counts/n) # | 11.3% (9/80) | 2.9% (1/35) | 6.9% (2/29) | 0.586 | 37.5% (6/16) |
| Treatment Groups | Number of Incidences | Percentage of Incidences with Triple Positive aPL %; (Counts) | Overall Length of Treatment Periods (Months) | Incidence/ 100 Patient Years 1 | Incidence Risk Ratio 1 | p-Value 1 |
|---|---|---|---|---|---|---|
| VKA | 6 | 33%; (2/6) | 3368 | 0.4 (0.1–2.4) | Reference | |
| DOAC | 12 | 67%; (8/12) | 1575 | 4.5 (1.5–13.3) | 10.4 (2.8–39.1) | <0.001 |
| Apixaban | 3 | 0%; (0/3) | 944 | 2.4 (0.6–6.6) | 3.4 (0.6–17.9) | 0.1518 |
| Rivaroxaban | 5 | 80%; (4/5) | 525 | 6.1 (1.6–23.0) | 8.5 (1.9–38.5) | 0.0058 |
| Edoxaban | 2 | 100%; (2/2) | 52 | n.a. 2 | ||
| Dabigatran | 2 | 100%; (2/2) | 54 | |||
| s.c. anticoagulation 3 | 5 | 100%; (5/5) | 338 | 6.2 (1.3–29.8) | 14.3 (3.0–67.2) | <0.001 |
| Antiplatelet drug | 6 | 0%; (0/6) | 2059 | 1.3 (0.3–5.9) | 3.1 (0.7–13.4) | 0.1329 |
| s.c. anticoagulation and antiplatelet drug | 2 | 0%; (0/2) | 189 | 2.7 (0.3–24.5) | 6.1 (0.8–49.0) | 0.0872 |
| Treatment Groups | Number of Incidences | Percentage of Incidences with Triple Positive aPL %; (Counts) | Overall Length of Treatment Periods (Months) | Incidence/ 100 Patient Years 1 | Incidence Risk Ratio 1 | p-Value 1 |
|---|---|---|---|---|---|---|
| VKA | 10 | 80%; (8/10) | 3368 | 1.6 (0.6–4.5) | Ref. | |
| DOAC | 5 | 80%; (4/5) | 1575 | 1.5 (0.4–5.1) | 0.9 (0.3–3.3) | 0.9086 |
| Apixaban | 3 | 67%; (2/3) | 944 | 1.6 (0.4–6.6) | 1.0 (0.2–5.2) | 0.9634 |
| Rivaroxaban | 2 | 100%; (2/2) | 525 | 1.5 (0.2–12.4) | 1.0 (0.1–9.2) | 0.9917 |
| Edoxaban | 0 | 0 | 52 | n.a. 2 | ||
| Dabigatran | 0 | 0 | 54 | |||
| s.c. anticoagulation 3 | 5 | 40%; (2/5) | 338 | 5.9 (1.4–25.2) | 3.7 (1.0–14.5) | 0.0581 |
| Antiplatelet drug | 13 | 23%; (3/13) | 2059 | 4.8 (1.9–11.7) | 3.0 (1.0–8.8) | 0.0471 |
| s.c. anticoagulation and antiplatelet drug | 1 | 100%; (1/1) | 189 | 3.8 (0.4–34.5) | 2.4 (0.2–23.1) | 0.4530 |
| Treatment Groups | Number of Incidences | Overall Length of Treatment Periods (Months) | Incidence/ 100 Patient Years 1 | Incidence Risk Ratio 1 | p-Value 1 |
|---|---|---|---|---|---|
| VKA | 8 | 3368 | 1.6 (0.5–4.9) | Reference | |
| DOAC | 8 | 1575 | 2.6 (0.7–9.0) | 1.6 (0.5–5.1) | 0.4536 |
| Apixaban | 5 | 944 | 2.5 (0.6–10.7) | 1.6 (0.4–6.5) | 0.4812 |
| Rivaroxaban | 2 | 525 | 1.4 (0.2–9.3) | 0.9 (0.2–5.4) | 0.9359 |
| Edoxaban | 1 | 52 | n.a. 2 | ||
| Dabigatran | 0 | 54 | |||
| s.c. anticoagulation 3 | 3 | 338 | 4.0 (0.7–23.0) | 2.4 (0.4–13.3) | 0.3053 |
| Antiplatelet drug | 2 | 2059 | 0.3 (0.0–2.4) | 0.2 (0.0–1.2) | 0.0733 |
| s.c. anticoagulation and antiplatelet drug | 2 | 189 | 6.9 (1.1–43.5) | 4.3 (0.7–27.4) | 0.1276 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, A.; Herrmann, E.; Ott, O.; Lindhoff-Last, E. Thromboembolic Antiphospholipid Syndrome (APS): Efficacy and Safety of Different Anticoagulants-Results of the APSantiCO Registry. J. Clin. Med. 2022, 11, 4845. https://doi.org/10.3390/jcm11164845
Schulz A, Herrmann E, Ott O, Lindhoff-Last E. Thromboembolic Antiphospholipid Syndrome (APS): Efficacy and Safety of Different Anticoagulants-Results of the APSantiCO Registry. Journal of Clinical Medicine. 2022; 11(16):4845. https://doi.org/10.3390/jcm11164845
Chicago/Turabian StyleSchulz, Annabel, Eva Herrmann, Olivia Ott, and Edelgard Lindhoff-Last. 2022. "Thromboembolic Antiphospholipid Syndrome (APS): Efficacy and Safety of Different Anticoagulants-Results of the APSantiCO Registry" Journal of Clinical Medicine 11, no. 16: 4845. https://doi.org/10.3390/jcm11164845
APA StyleSchulz, A., Herrmann, E., Ott, O., & Lindhoff-Last, E. (2022). Thromboembolic Antiphospholipid Syndrome (APS): Efficacy and Safety of Different Anticoagulants-Results of the APSantiCO Registry. Journal of Clinical Medicine, 11(16), 4845. https://doi.org/10.3390/jcm11164845







