Anticoagulant Therapy in Patients with Antiphospholipid Syndrome
Abstract
:1. Introduction
2. Epidemiology
3. Physiopathology of Thrombosis in APS
4. Diagnosis
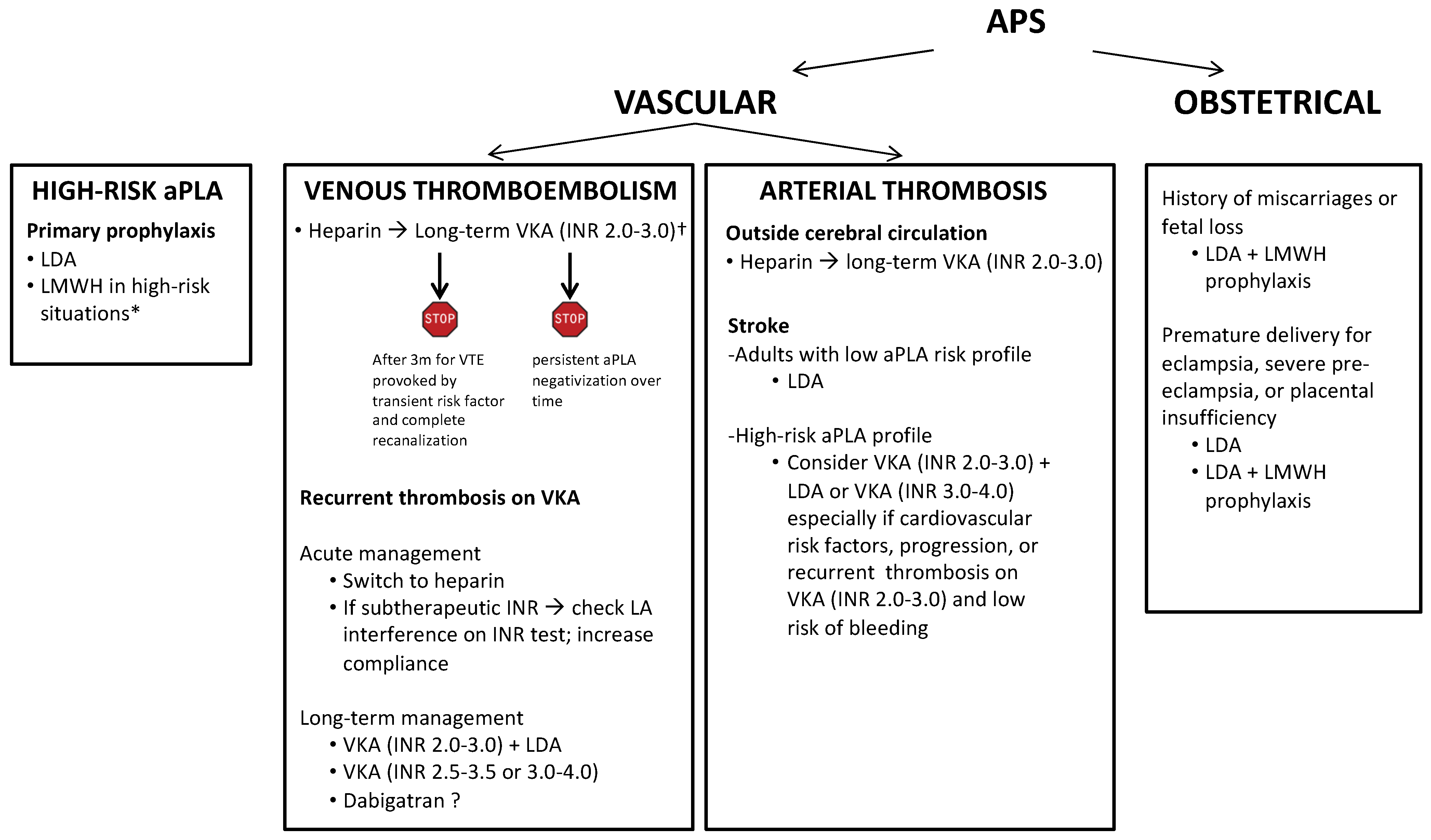
5. Primary Antithrombotic Prophylaxis
6. Treatment of Venous Thromboembolism
7. Treatment of Arterial Thrombosis
8. Secondary Antithrombotic Prophylaxis
9. Direct Oral Anticoagulants
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chayoua, W.; Kelchtermans, H.; Moore, G.W.; Musiał, J.; Wahl, D.; de Laat, B.; Devreese, K.M.J. Identification of high thrombotic risk triple-positive antiphospholipid syndrome patients is dependent on anti-cardiolipin and anti-β2glycoprotein I antibody detection assays. J. Thromb. Haemost. 2018, 16, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Yin, D.; De Groot, P.G.; Ninivaggi, M.; Devreese, K.M.J.; De Laat, B. Clinical relevance of isolated lupus anticoagulant positivity in patients with thrombotic antiphospholipid syndrome. Thromb. Haemost. 2021, 121, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.R.V. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. Br. Med. J. 1983, 287, 1088–1089. [Google Scholar] [CrossRef] [Green Version]
- Asherson, R.A.; Mackworth-Young, C.G.; Harris, E.N.; Gharavi, A.E.; Hughes, G.R.V. Multiple venous and arterial thromboses associated with the lupus anticoagulant and antibodies to cardiolipin in the absence of SLE. Rheumatol. Int. 1985, 5, 91–93. [Google Scholar] [CrossRef]
- Harris, E.N. Syndrome of the black swan. Rheumatology 1987, 26, 324–326. [Google Scholar] [CrossRef] [Green Version]
- Duarte-García, A.; Pham, M.M.; Crowson, C.S.; Amin, S.; Moder, K.G.; Pruthi, R.K.; Warrington, K.J.; Matteson, E.L. The Epidemiology of Antiphospholipid Syndrome: A Population-Based Study. Arthritis Rheumatol. 2019, 71, 1545–1552. [Google Scholar] [CrossRef]
- Radin, M.; Sciascia, S.; Bazzan, M.; Bertero, T.; Carignola, R.; Montabone, E.; Montaruli, B.; Vaccarino, A.; Cecchi, I.; Rubini, E.; et al. Antiphospholipid Syndrome Is Still a Rare Disease—Estimated Prevalence in the Piedmont and Aosta Valley Regions of Northwest Italy: Comment on the Article by Duarte-García et al. Arthritis Rheumatol. 2020, 72, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.-C.; Leong, K.-H.; Chiu, L.-T.; Chou, P.-Y.; Wu, L.-C.; Chou, C.-Y.; Kuo, C.-F.; Tsai, S.-Y. The trends in the incidence and thrombosis-related comorbidities of antiphospholipid syndrome: A 14-year nationwide population-based study. Thromb. J. 2022, 20, 1–9. [Google Scholar] [CrossRef]
- Vila, P.; Hernández, M.C.; López-Fernández, M.F.; Batlle, J. Prevalence, follow-up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb. Haemost. 1994, 72, 209–213. [Google Scholar] [CrossRef]
- Petri, M. Epidemiology of the antiphospholipid antibody syndrome. J. Autoimmun. 2000, 15, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Park, J.; Le Gal, G.; Piran, S.; Kherani, S.; Rodger, M.A.; Delluc, A. Prevalence of confirmed antiphospholipid syndrome in 18–50 years unselected patients with first unprovoked venous thromboembolism. J. Thromb. Haemost. 2020, 18, 926–930. [Google Scholar] [CrossRef]
- Pengo, V.; Testa, S.; Martinelli, I.; Ghirarduzzi, A.; Legnani, C.; Gresele, P.; Passamonti, S.M.; Bison, E.; Denas, G.; Jose, S.P.; et al. Incidence of a first thromboembolic event in carriers of isolated lupus anticoagulant. Thromb. Res. 2015, 135, 46–49. [Google Scholar] [CrossRef]
- Mustonen, P.; Lehtonen, K.V.; Javela, K.; Puurunen, M. Persistent antiphospholipid antibody (aPL) in asymptomatic carriers as a risk factor for future thrombotic events: A nationwide prospective study. Lupus 2014, 23, 1468–1476. [Google Scholar] [CrossRef]
- Kelchtermans, H.; Pelkmans, L.; de Laat, B.; Devreese, K.M. IgG/IgM antiphospholipid antibodies present in the classification criteria for the antiphospholipid syndrome: A critical review of their association with thrombosis. J. Thromb. Haemost. 2016, 14, 1530–1548. [Google Scholar] [CrossRef]
- Moschetti, L.; Dal Pozzolo, L.; Le Guern, V.; Morel, N.; Yelnik, C.M.; Lambert, M.; Hachulla, E.; Benhamou, Y.; Nalli, C.; Fredi, M.; et al. Gender differences in primary antiphospholipid syndrome with vascular manifestations in 433 patients from four European centres. Clin. Exp. Rheumatol. 2022, 40, S19–S26. [Google Scholar] [CrossRef]
- Cervera, R.; Piette, J.; Font, J.; Khamashta, M.; Shoenfeld, Y.; Camps, M.; Jacobsen, S.; Lakos, G.; Tincani, A.; Kontopoulou-Griva, I.; et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients. Arthritis Rheumatol. 2002, 46, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pintó, I.; Moitinho, M.; Santacreu, I.; Shoenfeld, Y.; Erkan, D.; Espinosa, G.; Cervera, R. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun. Rev. 2016, 15, 1120–1124. [Google Scholar] [CrossRef]
- Bucciarelli, S.; Espinosa, G.; Cervera, R.; Erkan, D.; Gómez-Puerta, J.A.; Ramos-Casals, M.; Font, J.; Asherson, R.A. Mortality in the catastrophic antiphospholipid syndrome: Causes of death and prognostic factors in a series of 250 patients. Arthritis Rheumatol. 2006, 54, 2568–2576. [Google Scholar] [CrossRef]
- Rodríguez-Pintó, I.; Espinosa, G.; Erkan, D.; Shoenfeld, Y.; Cervera, R.; Cervera, R.; Espinosa, G.; Rodríguez-Pintó, I.; Shoenfeld, Y.; Erkan, D.; et al. The effect of triple therapy on the mortality of catastrophic anti-phospholipid syndrome patients. Rheumatology 2018, 57, 1264–1270. [Google Scholar] [CrossRef] [Green Version]
- Bertero, M.; Bazzan, M.; Carignola, R.; Montaruli, B.; Silvestro, E.; Sciascia, S.; Vaccarino, A.; Baldovino, S.; Roccatello, D. Antiphospholipid syndrome in northwest Italy (APS Piedmont Cohort): Demographic features, risk factors, clinical and laboratory profile. Lupus 2012, 21, 806–809. [Google Scholar] [CrossRef]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; De Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef]
- Pengo, V.; Ruffatti, A.; Legnani, C.; Gresele, P.; Barcellona, D.; Erba, N.; Testa, S.; Marongiu, F.; Bison, E.; Denas, G.; et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J. Thromb. Haemost. 2010, 8, 237–242. [Google Scholar] [CrossRef]
- Niznik, S.; Rapoport, M.J.; Avnery, O.; Lubetsky, A.; Haj Yahia, S.; Ellis, M.H.; Agmon-Levin, N. Patterns of Recurrent Thrombosis in Primary Antiphospholipid Syndrome—Multicenter, Real-Life Long-Term Follow-Up. Front. Immunol. 2022, 13, 843718. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Sebastiani, G.D.; Iuliano, A.; Cantarini, L.; Galeazzi, M. Genetic aspects of the antiphospholipid syndrome: An update. Autoimmun. Rev. 2016, 15, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Betapudi, V.; Lominadze, G.; Hsi, L.; Willard, B.; Wu, M.; McCrae, K.R. Anti-β2GPI antibodies stimulate endothelial cell microparticle release via a nonmuscle myosin II motor protein-dependent pathway. Blood 2013, 122, 3808–3817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liestøl, S.; Sandset, P.M.; Jacobsen, E.M.; Mowinckel, M.-C.; Wisløff, F. Decreased anticoagulant response to tissue factor pathway inhibitor type 1 in plasmas from patients with lupus anticoagulants. Br. J. Haematol. 2007, 136, 131–137. [Google Scholar] [CrossRef]
- Arachchillage, D.R.J.; Efthymiou, M.; Mackie, I.J.; Lawrie, A.S.; Machin, S.J.; Cohen, H. Anti-protein C antibodies are associated with resistance to endogenous protein C activation and a severe thrombotic phenotype in antiphospholipid syndrome. J. Thromb. Haemost. 2014, 12, 1801–1809. [Google Scholar] [CrossRef]
- Romay-Penabad, Z.; Montiel-Manzano, M.G.; Shilagard, T.; Papalardo, E.; Vargas, G.; Deora, A.B.; Wang, M.; Jacovina, A.T.; Garcia-Latorre, E.; Reyes-Maldonado, E.; et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood 2009, 114, 3074–3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gropp, K.; Weber, N.; Reuter, M.; Micklisch, S.; Kopka, I.; Hallström, T.; Skerka, C. β2-glycoprotein I, the major target in antiphospholipid syndrome, is a special human complement regulator. Blood 2011, 118, 2774–2783. [Google Scholar] [CrossRef]
- Årfors, L.; Lefvert, A.K. Enrichment of antibodies against phospholipids in circulating immune complexes (CIC) in the anti-phospholipid syndrome (APLS). Clin. Exp. Immunol. 2003, 108, 47–51. [Google Scholar] [CrossRef]
- Müller-Calleja, N.; Hollerbach, A.; Ritter, S.; Pedrosa, D.G.; Strand, D.; Graf, C.; Reinhardt, C.; Strand, S.; Poncelet, P.; Griffin, J.H.; et al. Tissue factor pathway inhibitor primes monocytes for antiphospholipid antibody-induced thrombosis. Blood 2019, 134, 1119–1131. [Google Scholar] [CrossRef]
- Proulle, V.; Furie, R.A.; Merrill-Skoloff, G.; Furie, B.C.; Furie, B. Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood 2014, 124, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Yalavarthi, S.; Gould, T.J.; Rao, A.N.; Mazza, L.F.; Morris, A.E.; Núñez-Álvarez, C.; Hernández-Ramírez, D.; Bockenstedt, P.L.; Liaw, P.C.; Cabral, A.R.; et al. Release of Neutrophil Extracellular Traps by Neutrophils Stimulated with Antiphospholipid Antibodies: A Newly Identified Mechanism of Thrombosis in the Antiphospholipid Syndrome. Arthritis Rheumatol. 2015, 67, 2990–3003. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Gudjonsson, J.E.; Kahlenberg, J.M.; Joseph McCune, W.; Bockenstedt, P.L.; Karp, D.R.; Knight, J.S. Anti–Neutrophil Extracellular Trap Antibodies and Impaired Neutrophil Extracellular Trap Degradation in Antiphospholipid Syndrome. Arthritis Rheumatol. 2020, 72, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Mazetto, B.D.M.; Hounkpe, B.W.; da Silva Saraiva, S.; Vieira-Damiani, G.; dos Santos, A.P.R.; Jacinto, B.C.; Vaz, C.D.O.; Mesquita, G.T.V.; Annichino-Bizzacchi, J.M.; De Paula, E.V.; et al. Association between neutrophil extracellular traps (NETs) and thrombosis in antiphospholipid syndrome. Thromb. Res. 2022, 214, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dong, Y.; Zhang, Y.; Shen, D.; Wang, X.; Ge, R.; Zhang, M.; Xia, Y.; Wang, X. Antiphospholipid antibody-activated NETs exacerbate trophoblast and endothelial cell injury in obstetric antiphospholipid syndrome. J. Cell. Mol. Med. 2020, 24, 6690–6703. [Google Scholar] [CrossRef] [PubMed]
- Leffler, J.; Stojanovich, L.; Shoenfeld, Y.; Bogdanovic, G.; Hesselstrand, R.; Blom, A.M. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin. Exp. Rheumatol. 2014, 32, 66–70. [Google Scholar]
- López-Pedrera, C.; Buendía, P.; José Cuadrado, M.; Siendones, E.; Angeles Aguirre, M.; Barbarroja, N.; Montiel-Duarte, C.; Torres, A.; Khamashta, M.; Velasco, F. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-κB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK path. Arthritis Rheumatol. 2006, 54, 301–311. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Erkan, D.; Espinosa, G.; Cervera, R. Catastrophic antiphospholipid syndrome: Updated diagnostic algorithms. Autoimmun. Rev. 2010, 10, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, L.; Liu, H.; Cai, Q. Non-criteria antiphospholipid antibodies in antiphospholipid syndrome: Diagnostic value added. Front. Immunol. 2022, 13, 972012. [Google Scholar] [CrossRef] [PubMed]
- Otomo, K.; Atsumi, T.; Amengual, O.; Fujieda, Y.; Kato, M.; Oku, K.; Horita, T.; Yasuda, S.; Koike, T. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheumatol. 2012, 64, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Sanna, G.; Murru, V.; Roccatello, D.; Khamashta, M.A.; Bertolaccini, M.L. GAPSS: The global anti-phospholipid syndrome score. Rheumatology 2013, 52, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, L.; Mathian, A.; Ruffatti, A.; Erkan, D.; Tektonidou, M.; Cervera, R.; Forastiero, R.; Pengo, V.; Lambert, M.; Martinez-Zamora, M.A.; et al. Efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies: An international and collaborative meta-analysis. Autoimmun. Rev. 2014, 13, 281–291. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Laskari, K.; Panagiotakos, D.B.; Moutsopoulos, H.M. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Care Res. 2009, 61, 29–36. [Google Scholar] [CrossRef]
- Amengual, O.; Fujita, D.; Ota, E.; Carmona, L.; Oku, K.; Sugiura-Ogasawara, M.; Murashima, A.; Atsumi, T. Primary prophylaxis to prevent obstetric complications in asymptomatic women with antiphospholipid antibodies: A systematic review. Lupus 2015, 24, 1135–1142. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Bueno, H.; Galié, N.; Gibbs, J.S.R.; Ageno, W.; Agewall, S.; Almeida, A.G.; Andreotti, F.; Barbato, E.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [Green Version]
- Bates, S.M.; Middeldorp, S.; Rodger, M.; James, A.H.; Greer, I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 92–128. [Google Scholar] [CrossRef] [Green Version]
- Søgaard, M.; Skjøth, F.; Nielsen, P.B.; Beyer-Westendorf, J.; Larsen, T.B. First Trimester Anticoagulant Exposure and Adverse Pregnancy Outcomes in Women with Preconception Venous Thromboembolism: A Nationwide Cohort Study. Am. J. Med. 2022, 135, 493–502.e5. [Google Scholar] [CrossRef] [PubMed]
- Yarrington, C.D.; Valente, A.M.; Economy, K.E. Cardiovascular management in pregnancy. Circulation 2015, 132, 1354–1364. [Google Scholar] [CrossRef] [Green Version]
- Levine, S.R. Antiphospholipid Antibodies and Subsequent Thrombo-occlusive Events in Patients with Ischemic Stroke. JAMA 2004, 291, 576–584. [Google Scholar] [PubMed] [Green Version]
- Crowther, M.; Ginsberg, J.S.; Julian, J.; Denburg, J.; Hirsh, J.; Douketis, J.; Laskin, C.; Fortin, P.; Anderson, D.; Kearon, C.; et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N. Engl. J. Med. 2003, 349, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; Marchioli, R.; Brancaccio, V.; Schinco, P.; Wisloff, F.; Musial, J.; Baudo, F.; Berrettini, M.; Testa, S.; D’Angelo, A.; et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J. Thromb. Haemost. 2005, 3, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Akl, E.A.; Carr, R.; Kearon, C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: A systematic review. Blood 2013, 122, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Cao, S.; Yu, B.; He, T. Comparing the efficacy and safety of direct oral anticoagulants versus Vitamin K antagonists in patients with antiphospholipid syndrome: A systematic review and meta-analysis. Blood Coagul. Fibrinolysis 2022, 33, 389–401. [Google Scholar] [CrossRef]
- Rosborough, T.K.; Shepherd, M.F. Unreliability of international normalized ratio for monitoring warfarin therapy in patients with lupus anticoagulant. Pharmacotherapy 2004, 24, 838–842. [Google Scholar] [CrossRef]
- Moll, S. Monitoring Warfarin Therapy in Patients with Lupus Anticoagulants. Ann. Intern. Med. 1997, 127, 177–185. [Google Scholar] [CrossRef]
- Kasthuri, R.S.; Roubey, R.A.S. Warfarin and the antiphospholipid syndrome: Does one size fit all? Arthritis Care Res. 2007, 57, 1346–1347. [Google Scholar] [CrossRef]
- Cohen, H.; Hunt, B.J.; Efthymiou, M.; Arachchillage, D.R.J.; Mackie, I.J.; Clawson, S.; Sylvestre, Y.; Machin, S.J.; Bertolaccini, M.L.; Ruiz-Castellano, M.; et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): A randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet. Haematol. 2016, 3, e426–e436. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Abbattista, M.; Bucciarelli, P.; Tripodi, A.; Artoni, A.; Gianniello, F.; Novembrino, C.; Peyvandi, F. Recurrent thrombosis in patients with antiphospholipid antibodies treated with vitamin K antagonists or rivaroxaban. Haematologica 2018, 103, e315–e316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pengo, V.; Denas, G.; Zoppellaro, G.; Jose, S.P.; Hoxha, A.; Ruffatti, A.; Andreoli, L.; Tincani, A.; Cenci, C.; Prisco, D.; et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018, 132, 1365–1371. [Google Scholar] [CrossRef]
- Pengo, V.; Hoxha, A.; Andreoli, L.; Tincani, A.; Silvestri, E.; Prisco, D.; Fierro, T.; Gresele, P.; Cafolla, A.; De Micheli, V.; et al. Trial of Rivaroxaban in AntiPhospholipid Syndrome (TRAPS): Two-year outcomes after the study closure. J. Thromb. Haemost. 2021, 19, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Ordi-Ros, J.; Sáez-Comet, L.; Pérez-Conesa, M.; Vidal, X.; Riera-Mestre, A.; Castro-Salomó, A.; Cuquet-Pedragosa, J.; Ortiz-Santamaria, V.; Mauri-Plana, M.; Solé, C.; et al. Rivaroxaban versus Vitamin K antagonist in antiphospholipid syndrome a randomized noninferiority trial. Ann. Intern. Med. 2019, 171, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Woller, S.C.; Stevens, S.M.; Kaplan, D.; Wang, T.F.; Ware Branch, D.; Groat, D.; Wilson, E.L.; Armbruster, B.; Aston, V.T.; Lloyd, J.F.; et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: A randomized trial. Blood Adv. 2022, 6, 1661–1670. [Google Scholar] [CrossRef]
- Perzborn, E.; Strassburger, J.; Wilmen, A.; Pohlmann, J.; Roehrig, S.; Schlemmer, K.H.; Straub, A. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—An oral, direct Factor Xa inhibitor. J. Thromb. Haemost. 2005, 3, 514–521. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Eriksson, H.; Kakkar, A.; Schellong, S.; Feuring, M.; Fraessdorf, M.; Kreuzer, J.; Schueler, E.; Schulman, S. Efficacy of dabigatran versus warfarin in patients with acute venous thromboembolism in the presence of thrombophilia: Findings from RE-COVER®, RE-COVER™ II, and RE-MEDY™. Vasc. Med. 2016, 21, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- McCormack, T.; Harrisingh, M.C.; Horner, D.; Bewley, S. Venous thromboembolism in adults: Summary of updated NICE guidance on diagnosis, management, and thrombophilia testing. BMJ 2020, 369, m1565. [Google Scholar] [CrossRef]
- Zuily, S.; Cohen, H.; Isenberg, D.; Woller, S.C.; Crowther, M.; Dufrost, V.; Wahl, D.; Doré, C.J.; Cuker, A.; Carrier, M.; et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: Guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2020, 18, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Cuadrado, M.J.; Erkan, D.; Duarte-Garcia, A.; Isenberg, D.A.; Knight, J.S.; Ortel, T.L.; Rahman, A.; Salmon, J.E.; Tektonidou, M.G.; et al. 16th International Congress on Antiphospholipid Antibodies Task Force Report on Antiphospholipid Syndrome Treatment Trends. Lupus 2020, 29, 1571–1593. [Google Scholar] [CrossRef] [PubMed]
- Beyer-Westendorf, J.; Tittl, L.; Bistervels, I.; Middeldorp, S.; Schaefer, C.; Paulus, W.; Thomas, W.; Kemkes-Matthes, B.; Marten, S.; Bornhauser, M. Safety of direct oral anticoagulant exposure during pregnancy: A retrospective cohort study. Lancet Haematol. 2020, 7, e884–e891. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capecchi, M.; Abbattista, M.; Ciavarella, A.; Uhr, M.; Novembrino, C.; Martinelli, I. Anticoagulant Therapy in Patients with Antiphospholipid Syndrome. J. Clin. Med. 2022, 11, 6984. https://doi.org/10.3390/jcm11236984
Capecchi M, Abbattista M, Ciavarella A, Uhr M, Novembrino C, Martinelli I. Anticoagulant Therapy in Patients with Antiphospholipid Syndrome. Journal of Clinical Medicine. 2022; 11(23):6984. https://doi.org/10.3390/jcm11236984
Chicago/Turabian StyleCapecchi, Marco, Maria Abbattista, Alessandro Ciavarella, Mario Uhr, Cristina Novembrino, and Ida Martinelli. 2022. "Anticoagulant Therapy in Patients with Antiphospholipid Syndrome" Journal of Clinical Medicine 11, no. 23: 6984. https://doi.org/10.3390/jcm11236984
APA StyleCapecchi, M., Abbattista, M., Ciavarella, A., Uhr, M., Novembrino, C., & Martinelli, I. (2022). Anticoagulant Therapy in Patients with Antiphospholipid Syndrome. Journal of Clinical Medicine, 11(23), 6984. https://doi.org/10.3390/jcm11236984




