Evaluation of the Correlation between Regional Retinal Ganglion Cell Damage and Visual Field Sensitivity in Patients with Advanced Glaucoma
Abstract
:1. Introduction
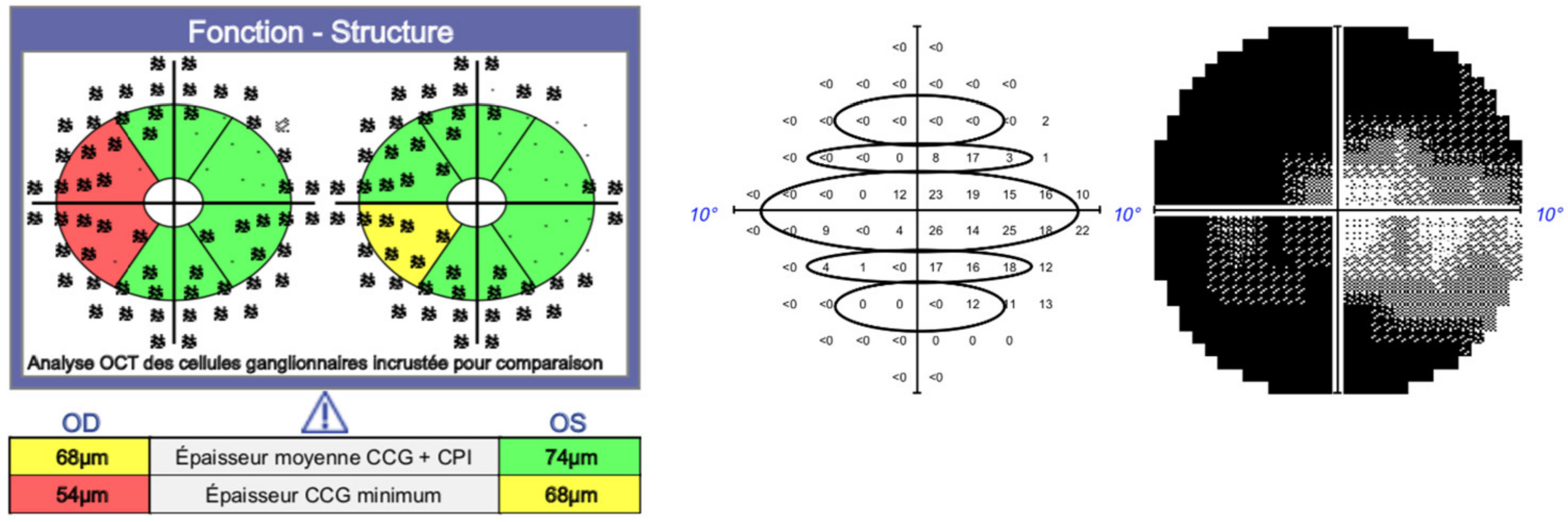
2. Materials and Methods
3. Results
3.1. Study Population
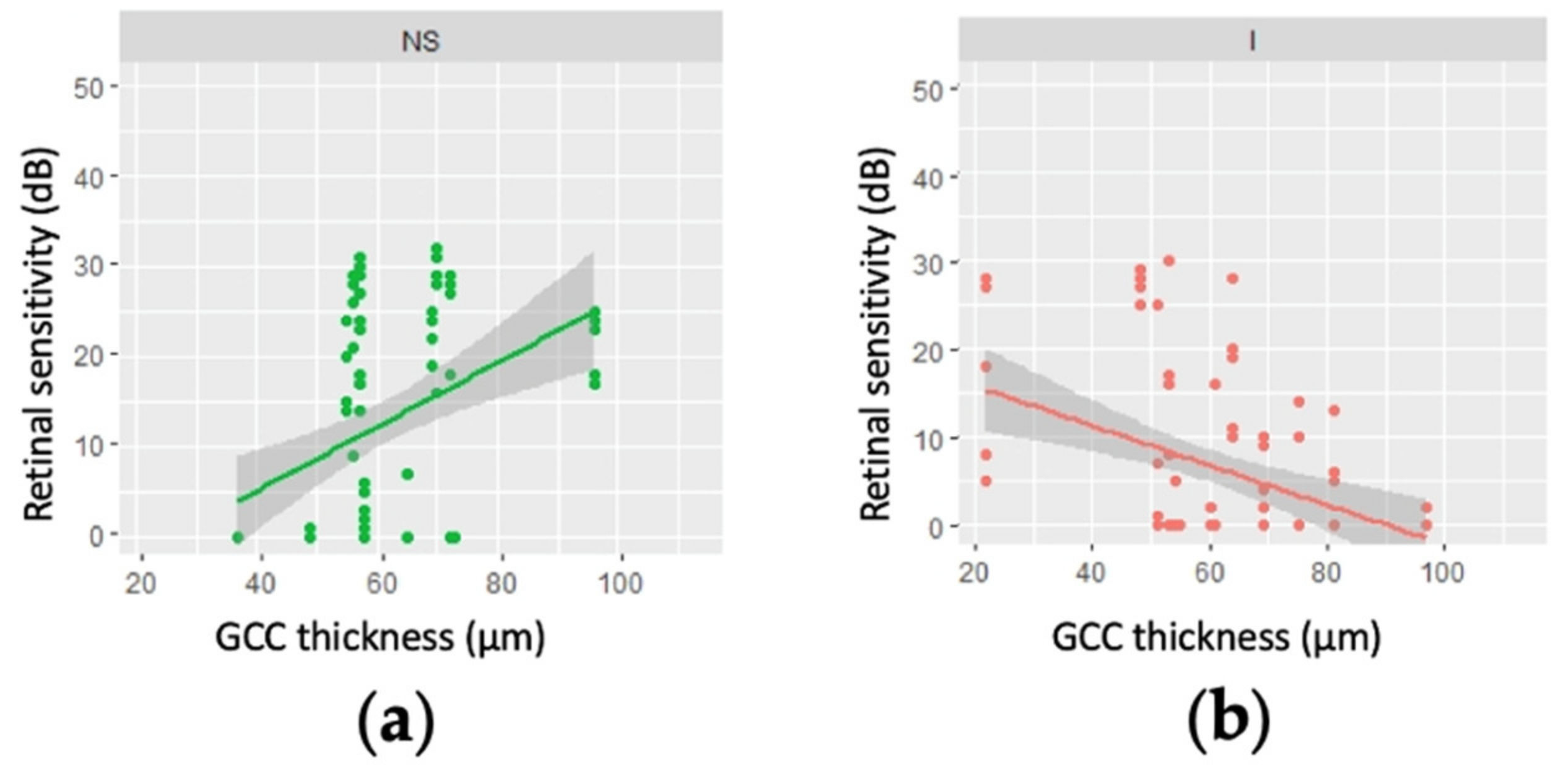
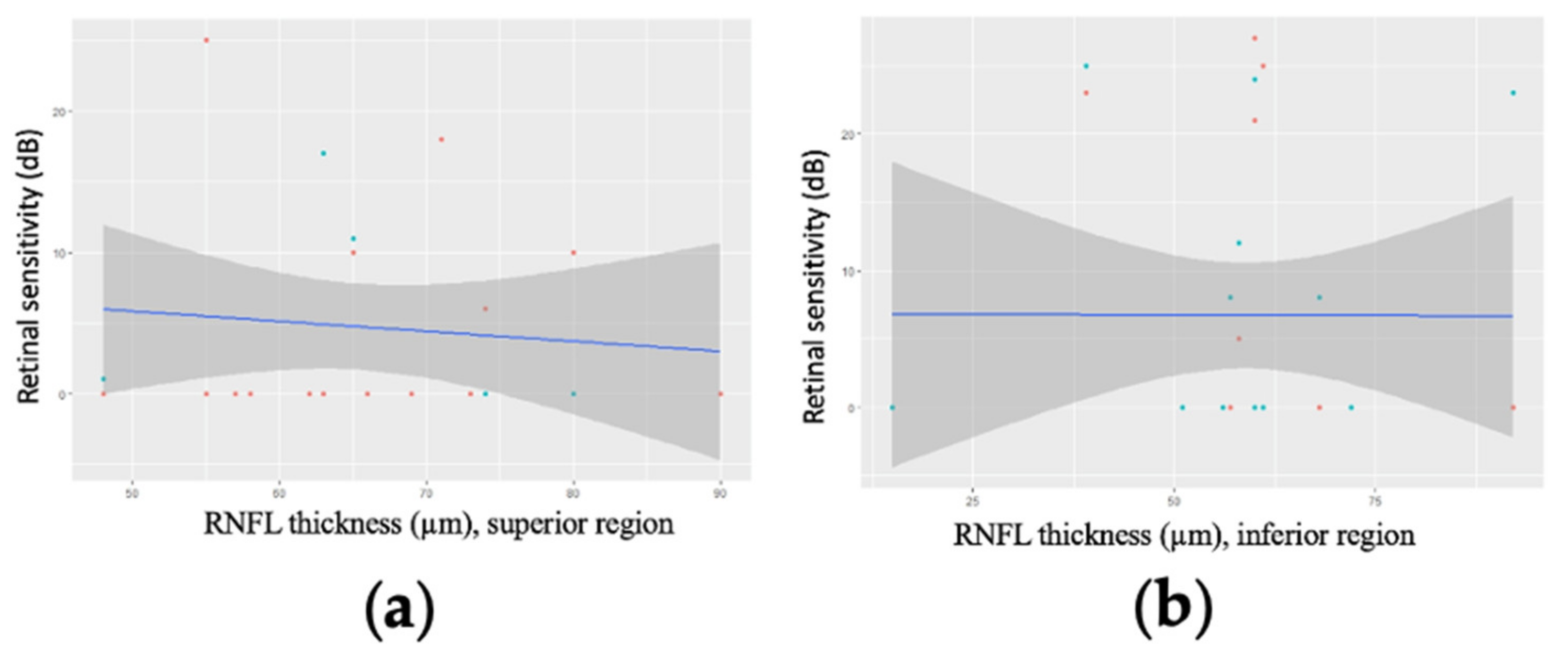
3.2. Correlation between GCC Thickness and Retinal Sensitivity
3.3. Correlation between Mean Regional GCC Thickness and Retinal Sensitivity for Patients with an MD Worse than −20 dB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition—Part 1Supported by the EGS Foundation. Br. J. Ophthalmol. 2017, 101, 1–72. [CrossRef]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline Factors That Predict the Onset of Primary Open-Angle Glaucoma. Arch. Ophthalmol. 2002, 120, 714–720, discussion 829–830. [Google Scholar] [CrossRef] [PubMed]
- Musch, D.C.; Lichter, P.R.; Guire, K.E.; Standardi, C.L. The Collaborative Initial Glaucoma Treatment Study: Study Design, Methods, and Baseline Characteristics of Enrolled Patients. Ophthalmology 1999, 106, 653–662. [Google Scholar] [CrossRef]
- Ederer, F.; Gaasterland, D.E.; Sullivan, E.K.; AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 1. Study Design and Methods and Baseline Characteristics of Study Patients. Control. Clin. Trials. 1994, 15, 299–325. [Google Scholar]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Early Manifest Glaucoma Trial Group Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Smith, C.A.; Chauhan, B.C. Imaging retinal ganglion cells: Enabling experimental technology for clinical application. Prog. Retin. Eye Res. 2014, 44, 1–14. [Google Scholar] [CrossRef]
- Ishikawa, H.; Stein, D.M.; Wollstein, G.; Beaton, S.; Fujimoto, J.G.; Schuman, J. Macular Segmentation with Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2005, 46, 2012–2017. [Google Scholar] [CrossRef]
- Sung, K.R.; Wollstein, G.; Kim, N.R.; Na, J.H.; Nevins, J.E.; Kim, C.Y.; Schuman, J. Macular assessment using optical coherence tomography for glaucoma diagnosis. Br. J. Ophthalmol. 2012, 96, 1452–1455. [Google Scholar] [CrossRef]
- Fankhauser, F.; Koch, P.; Roulier, A. On Automation of Perimetry. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1972, 184, 126–150. [Google Scholar] [CrossRef]
- Torres, L.A.; Hatanaka, M. Correlating Structural and Functional Damage in Glaucoma. J. Glaucoma 2019, 28, 1079–1085. [Google Scholar] [CrossRef]
- Hodapp, E.; Anderson, D.R.; Parrish, R.K. Clinical Decisions in Glaucoma; Mosby: St. Louis, MO, USA, 1993; ISBN 978-0-8016-6799-2. [Google Scholar]
- Aptel, F.; Sayous, R.; Fortoul, V.; Beccat, S.; Denis, P. Structure–Function Relationships Using Spectral-Domain Optical Coherence Tomography: Comparison with Scanning Laser Polarimetry. Am. J. Ophthalmol. 2010, 150, 825–833.e1. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Allen, K.A. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Sung, K.R.; Lee, G.C.; Durbin, M.K.; Cheng, D. Ganglion Cell–Inner Plexiform Layer Change Detected by Optical Coherence Tomography Indicates Progression in Advanced Glaucoma. Ophthalmology 2017, 124, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.S.; Heo, H.; Park, S.W. Structure-function Relationship in Advanced Glaucoma after Reaching the RNFL Floor. J. Glaucoma 2019, 28, 1006–1011. [Google Scholar] [CrossRef]
- Drasdo, N.; Millican, C.L.; Katholi, C.R.; Curcio, C.A. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vis. Res. 2007, 47, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Kardon, R.H. A framework for comparing structural and functional measures of glaucomatous damage. Prog. Retin. Eye Res. 2007, 26, 688–710. [Google Scholar] [CrossRef]
- Garway-Heath, D.F.; Poinoosawmy, D.; Fitzke, F.W.; Hitchings, R.A. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 2000, 107, 1809–1815. [Google Scholar] [CrossRef]
- Lamparter, J.; Russell, R.A.; Zhu, H.; Asaoka, R.; Yamashita, T.; Ho, T.; Garway-Heath, D.F. The Influence of Intersubject Variability in Ocular Anatomical Variables on the Mapping of Retinal Locations to the Retinal Nerve Fiber Layer and Optic Nerve Head. Investig. Opthalmol. Vis. Sci. 2013, 54, 6074–6082. [Google Scholar] [CrossRef]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.V.; Liebmann, J.M.; Ritch, R. Glaucomatous damage of the macula. Prog. Retin. Eye Res. 2013, 32, 1–21. [Google Scholar] [CrossRef]
- Hood, D.C. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog. Retin. Eye Res. 2016, 57, 46–75. [Google Scholar] [CrossRef]
- Hood, D.C.; Anderson, S.C.; Wall, M.; Kardon, R. Structure versus Function in Glaucoma: An Application of a Linear Model. Investig. Opthalmol. Vis. Sci. 2007, 48, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.S.; Cho, J.; de Moraes, C.G.V.; Wang, M.; Zhang, X.; Kardon, R.H.; Liebmann, J.M.; Ritch, R.; Hood, D.C. Retinal Ganglion Cell Layer Thickness and Local Visual Field Sensitivity in Glaucoma. Arch. Ophthalmol. 2011, 129, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Mwanza, J.-C.; Kim, H.Y.; Budenz, D.L.; Warren, J.L.; Margolis, M.; Lawrence, S.D.; Jani, P.D.; Thompson, G.S.; Lee, R.K. Residual and Dynamic Range of Retinal Nerve Fiber Layer Thickness in Glaucoma: Comparison of Three OCT Platforms. Investig. Opthalmol. Vis. Sci. 2015, 56, 6344–6351. [Google Scholar] [CrossRef]
- Mwanza, J.-C.; Budenz, D.L.; Warren, J.L.; Webel, A.D.; Reynolds, C.E.; Barbosa, D.T.; Lin, S. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br. J. Ophthalmol. 2014, 99, 732–737. [Google Scholar] [CrossRef]
- Banister, K.; Boachie, C.; Bourne, R.; Cook, J.; Burr, J.M.; Ramsay, C.; Garway-Heath, D.; Gray, J.; McMeekin, P.; Hernández, R.; et al. Can Automated Imaging for Optic Disc and Retinal Nerve Fiber Layer Analysis Aid Glaucoma Detection? Ophthalmology 2016, 123, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Radius, R.L.; Anderson, D.R. The Histology of Retinal Nerve Fiber Layer Bundles and Bundle Defects. Arch. Ophthalmol. 1979, 97, 948–950. [Google Scholar] [CrossRef]
- Ye, C.; Yu, M.; Leung, C. Impact of segmentation errors and retinal blood vessels on retinal nerve fibre layer measurements using spectral-domain optical coherence tomography. Acta Ophthalmol. 2015, 94, e211–e219. [Google Scholar] [CrossRef]
- Wang, L.; Cioffi, G.; Cull, G.; Dong, J.; Fortune, B. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1088–1094. [Google Scholar]
- Bowd, C.; Zangwill, L.M.; Weinreb, R.N.; Medeiros, F.A.; Belghith, A. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am. J. Ophthalmol. 2016, 175, 37–44. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Vargas, J.L.C.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Wall, M.; Woodward, K.R.; Doyle, C.K.; Artes, P. Repeatability of Automated Perimetry: A Comparison between Standard Automated Perimetry with Stimulus Size III and V, Matrix, and Motion Perimetry. Investig. Opthalmol. Vis. Sci. 2009, 50, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Artes, P.H.; Iwase, A.; Ohno, Y.; Kitazawa, Y.; Chauhan, B.C. Properties of perimetric threshold estimates from Full Threshold, SITA Standard, and SITA Fast strategies. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2654–2659. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezkallah, A.; Douma, I.; Bonjour, M.; Mathis, T.; Kodjikian, L.; Denis, P. Evaluation of the Correlation between Regional Retinal Ganglion Cell Damage and Visual Field Sensitivity in Patients with Advanced Glaucoma. J. Clin. Med. 2022, 11, 4880. https://doi.org/10.3390/jcm11164880
Rezkallah A, Douma I, Bonjour M, Mathis T, Kodjikian L, Denis P. Evaluation of the Correlation between Regional Retinal Ganglion Cell Damage and Visual Field Sensitivity in Patients with Advanced Glaucoma. Journal of Clinical Medicine. 2022; 11(16):4880. https://doi.org/10.3390/jcm11164880
Chicago/Turabian StyleRezkallah, Amina, Ikrame Douma, Maxime Bonjour, Thibaud Mathis, Laurent Kodjikian, and Philippe Denis. 2022. "Evaluation of the Correlation between Regional Retinal Ganglion Cell Damage and Visual Field Sensitivity in Patients with Advanced Glaucoma" Journal of Clinical Medicine 11, no. 16: 4880. https://doi.org/10.3390/jcm11164880






