Influence of Mild Thyroid Dysfunction on Outcomes after Off-Pump Coronary Artery Bypass Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient
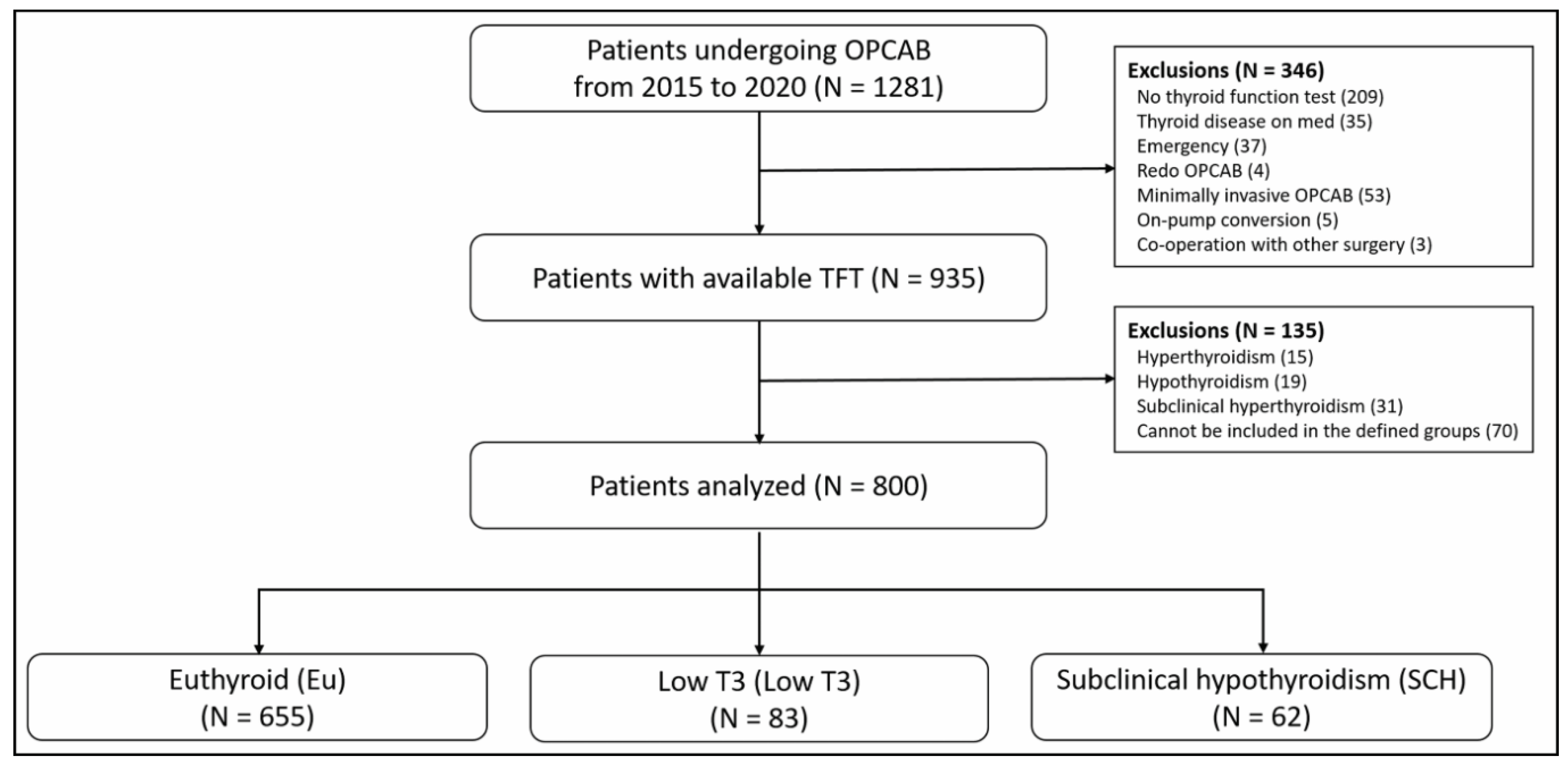
2.2. Thyroid Function Test and Patient Group Allocation
2.3. Anesthetic Management and Surgical Procedure
2.4. Study Endpoints and Variables
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Intraoperative Data
3.2. Analyses of the Primary and Secondary Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biancari, F.; Vasques, F.; Mikkola, R.; Martin, M.; Lahtinen, J.; Heikkinen, J. Validation of EuroSCORE II in Patients Undergoing Coronary Artery Bypass Surgery. Ann. Thorac. Surg. 2012, 93, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Ad, N.; Holmes, S.D.; Patel, J.; Pritchard, G.; Shuman, D.J.; Halpin, L. Comparison of EuroSCORE II, Original EuroSCORE, and the Society of Thoracic Surgeons Risk Score in Cardiac Surgery Patients. Ann. Thorac. Surg. 2016, 102, 573–579. [Google Scholar] [CrossRef]
- Cini, G.; Carpi, A.; Mechanick, J.; Cini, L.; Camici, M.; Galetta, F.; Giardino, R.; Russo, M.A.; Iervasi, G. Thyroid Hormones and the Cardiovascular System: Pathophysiology and Interventions. Biomed. Pharmacother. 2009, 63, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Rodondi, N.; den Elzen, W.P.; Bauer, D.C.; Cappola, A.R.; Razvi, S.; Walsh, J.P.; Asvold, B.O.; Iervasi, G.; Imaizumi, M.; Collet, T.H.; et al. Subclinical Hypothyroidism and the Risk of Coronary Heart Disease and Mortality. JAMA 2010, 304, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.S.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid Hormones and Cardiovascular Disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Razvi, S.; Pearce, S.H.; Dayan, C.M. Clinical Review: A Review of the Clinical Consequences of Variation in Thyroid Function within the Reference Range. J. Clin. Endocrinol. Metab. 2013, 98, 3562–3571. [Google Scholar] [CrossRef]
- Razvi, S. Novel Uses of Thyroid Hormones in Cardiovascular Conditions. Endocrine 2019, 66, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Iervasi, G.; Molinaro, S.; Landi, P.; Taddei, M.C.; Galli, E.; Mariani, F.; L’Abbate, A.; Pingitore, A. Association Between Increased Mortality and Mild Thyroid Dysfunction in Cardiac Patients. Arch. Intern. Med. 2007, 167, 1526–1532. [Google Scholar] [CrossRef]
- Molinaro, S.; Iervasi, G.; Lorenzoni, V.; Coceani, M.; Landi, P.; Srebot, V.; Mariani, F.; L’Abbate, A.; Pingitore, A. Persistence of Mortality Risk in Patients With Acute Cardiac Diseases and Mild Thyroid Dysfunction. Am. J. Med. Sci. 2012, 343, 65–70. [Google Scholar] [CrossRef]
- Seo, S.M.; Koh, Y.-S.; Park, H.-J.; Kim, D.B.; Her, S.H.; Lee, J.M.; Park, C.S.; Kin, P.-J.; Kim, H.Y.; Yoo, K.D.; et al. Thyroid stimulating hormone elevation as a predictor of long-term mortality in patients with acute myocardial infarction. Clin. Cardiol. 2018, 41, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Lim, Y.H.; Shin, J.H.; Park, J.; Shin, J. Impact of Subclinical Hypothyroidism on Clinical Outcomes Following Percutaneous Coronary Intervention. Int. J. Cardiol. 2018, 253, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Jiao, Y.; Yu, T.; Sun, Z. Association Between Mild Thyroid Dysfunction and Clinical Outcome in Acute Coronary Syn drome Undergoing Percutaneous Coronary Intervention. Cardiol. J. 2020, 27, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Cerillo, A.G.; Storti, S.; Kallushi, E.; Haxhiademi, D.; Miceli, A.; Murzi, M.; Berti, S.; Glauber, M.; Clerico, A.; Iervasi, G. The Low Triiodothyronine Syndrome: A Strong Predictor of Low Cardiac Output and Death in Patients Undergoing Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2014, 97, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Yoon, J.W.; Kim, K.I.; Lee, Y.J.; Kim, K.W.; Choi, S.H.; Lim, S.; Choi, D.J.; Park, K.H.; Choh, J.H.; et al. Subclinical Hypo thyroidism Might Increase the Risk of Transient Atrial Fibrillation after Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2009, 87, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Farina, P.; Gaudino, M.; Angelini, G.D. Off-Pump Coronary Artery Bypass Surgery: The Long and Winding Road. Int. J. Cardiol. 2019, 279, 51–55. [Google Scholar] [CrossRef]
- Takayama, T.; Hiro, T.; Hirayama, A. Is Angioplasty Able to Become the Gold Standard of Treatment Beyond Bypass Surgery for Patients with Multivessel Coronary Artery Disease? Therapeutic Strategies for 3-Vessel Coronary Artery Disease: OPCAB vs PCI(PCI-Side). Circ. J. 2010, 74, 2744–2749. [Google Scholar] [CrossRef]
- Song, Y.; Kwak, Y.L.; Song, J.W.; Kim, Y.J.; Shim, J.K. Respirophasic Carotid Artery Peak Velocity Variation as a Predictor of Fluid Responsiveness in Mechanically Ventilated Patients With Coronary Artery Disease. Br. J. Anaesth. 2014, 113, 61–66. [Google Scholar] [CrossRef]
- Goeddel, L.A.; Hopkins, A.N.; Fernando, R.J.; Núñez-Gil, I.J.; Ramakrishna, H. Analysis of the 4th Universal Definition of Myo cardial Infarction-Key Concepts and Perioperative Implications. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3486–3495. [Google Scholar] [CrossRef]
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Galli, E.; Pingitore, A.; Iervasi, G. The Role of Thyroid Hormone in the Pathophysiology of Heart Failure: Clinical Evidence. Heart Fail. Rev. 2010, 15, 155–169. [Google Scholar] [CrossRef]
- Nicolini, G.; Pitto, L.; Kusmic, C.; Balzan, S.; Sabatino, L.; Iervasi, G.; Forini, F. New Insights into Mechanisms of Cardioprotection Mediated by Thyroid Hormones. J. Thyroid Res. 2013, 2013, 264387. [Google Scholar] [CrossRef] [Green Version]
- Pantos, C.; Mourouzis, I.; Saranteas, T.; Clavé, G.; Ligeret, H.; Noack-Fraissignes, P.; Renard, P.Y.; Massonneau, M.; Perimenis, P.; Spanou, D.; et al. Thyroid Hormone Improves Postischaemic Recovery of Function While Limiting Apoptosis: A New Therapeutic Approach to Support Hemodynamics in the Setting of Ischaemia-Reperfusion? Basic Res. Cardiol. 2009, 104, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Scalise, P. Hypothyroidism and Failure to Wean in Patients Receiving Prolonged Mechanical Ventilation at a Regional Weaning Center. Chest 2004, 126, 1307–1312. [Google Scholar] [CrossRef]
- Zhang, M.; Sara, J.D.; Matsuzawa, Y.; Gharib, H.; Bell, M.R.; Gulati, R.; Lerman, L.O.; Lerman, A. Clinical Outcomes of Patients with Hypothyroidism Undergoing Percutaneous Coronary Intervention. Eur. Heart J. 2016, 37, 2055–2065. [Google Scholar] [CrossRef]
- Ning, Y.; Cheng, Y.J.; Liu, L.J.; Sara, J.D.; Cao, Z.Y.; Zheng, W.P.; Zhang, T.S.; Han, H.J.; Yang, Z.Y.; Zhang, Y.; et al. What Is the Association of Hypothyroidism with Risks of Cardiovascular Events and Mortality? A Meta-analysis of 55 Cohort Studies Involving 1,898,314 Participants. BMC Med. 2017, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Klein, I. Hypothyroidism as a Risk Factor for Cardiovascular Disease. Endocrine 2004, 24, 1–13. [Google Scholar] [CrossRef]
- Puskas, J.D.; Martin, J.; Cheng, D.C.; Benussi, S.; Bonatti, J.O.; Diegeler, A.; Ferdinand, F.D.; Kieser, T.M.; Lamy, A.; Mack, M.J.; et al. ISMICS Consensus Conference and Statements of Randomized Controlled Trials of Off-Pump Versus Conventional Coronary Artery Bypass Surgery. Innovations 2015, 10, 219–229. [Google Scholar] [CrossRef]
- Braverman, L.E.; Ingbar, S.H.; Sterling, K. Conversion of Thyroxine (T4) to Triiodothyronine (T3) in Athyreotic Human Subjects. J. Clin. Investig. 1970, 49, 855–864. [Google Scholar] [CrossRef]
- Cavalieri, R.R. Impaired Peripheral Conversion of Thyroxine to Triiodothyronine. Annu. Rev. Med. 1977, 28, 57–65. [Google Scholar] [CrossRef]
- Utiger, R.D. Altered Thyroid Function in Nonthyroidal Illness and Surgery. To Treat or Not to Treat? N. Engl. J. Med. 1995, 333, 1562–1563. [Google Scholar] [CrossRef]
- Forini, F.; Paolicchi, A.; Pizzorusso, T.; Ratto, G.M.; Saviozzi, M.; Vanini, V.; Iervasi, G. 3,5,3′-Triiodothyronine Deprivation Affects Phenotype and Intracellular [Ca2+]I of Human Cardiomyocytes in Culture. Cardiovasc. Res. 2001, 51, 322–330. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism: A Review. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Tseng, F.Y.; Lin, W.Y.; Lin, C.C.; Lee, L.T.; Li, T.C.; Sung, P.K.; Huang, K.C. Subclinical Hypothyroidism Is Associated with in creased Risk for All-Cause and Cardiovascular Mortality in Adults. J. Am. Coll. Cardiol. 2012, 60, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Türemen, E.E.; Çetinarslan, B.; Şahin, T.; Cantürk, Z.; Tarkun, İ. Endothelial Dysfunction and Low Grade Chronic Inflammation in Subclinical Hypothyroidism Due to Autoimmune Thyroiditis. Endocr. J. 2011, 58, 349–354. [Google Scholar] [CrossRef]
- Klemperer, J.D.; Klein, I.; Gomez, M.; Helm, R.E.; Ojamaa, K.; Thomas, S.J.; Isom, O.W.; Krieger, K. Thyroid Hormone Treatment after Coronary-Artery Bypass Surgery. N. Engl. J. Med. 1995, 333, 1522–1527. [Google Scholar] [CrossRef]
- Teiger, E.; Menasché, P.; Mansier, P.; Chevalier, B.; Lajeunie, E.; Bloch, G.; Piwnica, A. Triiodothyronine Therapy in Open-Heart Surgery: From Hope to Disappointment. Eur. Heart J. 1993, 14, 629–633. [Google Scholar] [CrossRef]

| Euthyroid (N = 655) | Low T3 (N = 83) | SCH (N = 62) | p-Value | |
|---|---|---|---|---|
| Age (years) | 65.4 ± 9.36 | 66.5 ± 9.95 | 67.4 ± 8.31 | 0.122 |
| Female | 137 (20.9) | 29 (34.9) | 13 (21.0) | 0.015 * |
| Body mass index (kg/m2) | 24.5 [22.6, 26.6] | 23.4 [21.1, 25.6] | 24.4 [22.6, 25.6] | 0.001 * |
| Hypertension | 462 (70.5) | 67 (80.7) | 46 (74.2) | 0.138 |
| Diabetes mellitus | 347 (53.0) | 57 (68.7) | 36 (58.1) | 0.022 * |
| Chronic kidney disease | 61 (9.3) | 45 (54.2) | 17 (27.4) | <0.001 *†‡ |
| Old cerebral infarction | 88 (13.4) | 12 (14.5) | 11 (17.7) | 0.636 |
| Atrial fibrillation | 14 (2.1) | 7 (8.4) | 8 (12.9) | <0.001 *† |
| COPD | 33 (5.0) | 4 (4.8) | 3 (4.8) | 0.994 |
| PAOD | 29 (4.4) | 9 (10.8) | 3 (4.8) | 0.044 * |
| Congestive heart failure | 37 (5.6) | 13 (15.7) | 8 (12.9) | 0.001 *† |
| Recent MI (<3 month) | 132 (20.2) | 23 (27.7) | 16 (25.8) | 0.193 |
| Acute coronary syndrome | 207 (31.6) | 45 (54.2) | 29 (46.8) | <0.001 *† |
| LVEF (%) | 59 [46, 67] | 45 [38, 57] | 54 [40, 63] | <0.001 * |
| Left main disease | 80 (12.2) | 10 (12.0) | 6 (9.7) | 0.841 |
| EuroSCORE | 1.46 [0.87, 3.00] | 2.21 [1.49, 3.98] | 1.83 [1.05, 3.07] | <0.001 * |
| Medications | ||||
| Beta blocker | 364 (55.6) | 43 (51.8) | 40 (64.5) | 0.292 |
| Calcium channel blocker | 273 (41.7) | 33 (39.8) | 26 (41.9) | 0.940 |
| ACEI/ARB | 375 (57.3) | 44 (53.0) | 32 (51.6) | 0.560 |
| Euthyroid (N = 655) | Low T3 (N = 83) | SCH (N = 62) | p-Value | |
|---|---|---|---|---|
| Laboratory data | ||||
| T3 (ng/mL) | 0.84 [0.74, 0.93] | 0.54 [0.45, 0.58] | 0.77 [0.68, 0.92] | <0.001 *†‡ |
| Free T4 (ng/dL) | 0.96 [0.89, 1.04] | 0.95 [0.88, 1.04] | 0.94 [0.87, 1.01] | 0.170 |
| TSH (µIU/mL) | 1.57 [1.03, 2.31] | 1.53 [0.91, 2.15] | 5.62 [4.82, 7.43] | <0.001 †‡ |
| Creatinine (mg/dL) | 0.87 [0.74, 1.04] | 1.33 [0.78, 4.72] | 0.95 [0.83, 1.34] | <0.001 *†‡ |
| Albumin (g/dL) | 4.1 [3.8, 4.4] | 3.5 [3.3, 3.8] | 3.8 [3.4, 4.2] | <0.001 *†‡ |
| Anemia | 252 (38.5) | 69 (83.1) | 41 (66.1) | <0.001 *†‡ |
| Troponin T (pg/mL) | 13.0 [8.0, 28.0] | 91.0 [21.0, 414.0] | 45.5 [13.0, 136.0] | <0.001 *‡ |
| Intraoperative data | ||||
| Anesthetic time (min) | 305 ± 41 | 299 ± 38 | 306 ± 37 | 0.402 |
| Operation time (min) | 235 ± 37 | 227 ± 35 | 233 ± 37 | 0.185 |
| Number of grafts | 3 [3, 4] | 3 [3, 4] | 3 [3, 4] | 0.151 |
| Euthyroid (N = 655) | Low T3 (N = 83) | SCH (N = 62) | p-Value | |
|---|---|---|---|---|
| Composite endpoints | 114 (17.4) | 42 (50.6) | 28 (45.2) | <0.001 *† |
| 30-day in-hospital mortality | 5 (0.8) | 4 (4.8) | 2 (3.2) | 0.005 * |
| Myocardial infarction | 5 (0.8) | 2 (2.4) | 2 (3.2) | 0.106 |
| Acute kidney injury | 97 (14.8) | 36 (43.4) | 24 (38.7) | <0.001 *† |
| Prolonged ventilator care over 24 h | 20 (3.1) | 6 (7.2) | 5 (8.1) | 0.037 † |
| Postoperative data | ||||
| Length of ICU stay (day) | 3 [2, 3] | 4 [3, 5] | 3 [3, 5] | <0.001 *† |
| Length of hospital stay (day) | 13 [11, 15] | 18 [13, 24] | 15 [12, 20] | <0.001 *† |
| Long-term all-cause mortality | 16 (2.4) | 8 (9.6) | 7 (11.3) | <0.001 *† |
| Cardiovascular | 6 (37.5) | 1 (12.5) | 4 (57.1) | |
| Multi-organ failure | 6 (37.5) | 7 (87.5) | 3 (42.9) | |
| Unspecified | 4 (25.0) | 0 | 0 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% C.I. | p-Value | OR | 95% C.I. | p-Value |
| Age | 1.037 | (1.017–1.057) | <0.001 | 1.028 | (1.007–1.049) | 0.010 |
| Female | 1.620 | (1.123–2.338) | 0.010 | |||
| Body mass index | 0.926 | (0.881–0.974) | 0.003 | |||
| Hypertension | 1.425 | (0.978–2.074) | 0.065 | |||
| Atrial fibrillation | 1.407 | (0.629–3.139) | 0.407 | |||
| CKD | 5.534 | (3.691–8.297) | <0.001 | 2.677 | (1.671–4.289) | <0.001 |
| CVA | 1.667 | (1.081–2.569) | 0.021 | |||
| Diabetes mellitus | 1.924 | (1.373–2.696) | <0.001 | |||
| COPD | 1.518 | (0.767–3.003) | 0.230 | |||
| PAOD | 1.644 | (0.844–3.202) | 0.144 | |||
| CHF | 1.555 | (0.876–2.760) | 0.131 | |||
| Recent MI | 1.526 | (1.050–2.219) | 0.027 | |||
| ACS | 1.269 | (0.910–1.769) | 0.161 | |||
| Left main disease | 1.239 | (0.769–1.997) | 0.379 | |||
| Hypoalbuminemia | 3.795 | (2.463–5.847) | <0.001 | |||
| Anemia | 3.354 | (2.385–4.715) | <0.001 | 2.029 | (1.375–2.995) | <0.001 |
| EuroSCORE | 1.196 | (1.119–1.277) | <0.001 | 1.138 | (1.057–1.225) | 0.001 |
| Low T3 | 4.301 | (2.682–6.896) | <0.001 | 2.294 | (1.330–3.955) | 0.003 |
| SCH | 3.458 | (2.022–5.913) | <0.001 | 2.845 | (1.582–5.116) | <0.001 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% C.I. | p-Value | HR | 95% C.I. | p-Value |
| EuroSCORE | 1.143 | (1.021–1.279) | 0.020 | |||
| Low T3 | 4.467 | (1.905–110.472) | 0.001 | 3.909 | (1.603–19.534) | 0.003 |
| SCH | 4.774 | (1.963–111.608) | 0.001 | 4.807 | (1.977–111.690) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joe, Y.-E.; Shin, Y.R.; Kwak, Y.-L.; Shim, J.H.; Shon, Y.S.; Shim, J.-K. Influence of Mild Thyroid Dysfunction on Outcomes after Off-Pump Coronary Artery Bypass Surgery. J. Clin. Med. 2022, 11, 5033. https://doi.org/10.3390/jcm11175033
Joe Y-E, Shin YR, Kwak Y-L, Shim JH, Shon YS, Shim J-K. Influence of Mild Thyroid Dysfunction on Outcomes after Off-Pump Coronary Artery Bypass Surgery. Journal of Clinical Medicine. 2022; 11(17):5033. https://doi.org/10.3390/jcm11175033
Chicago/Turabian StyleJoe, Young-Eun, Yu Rim Shin, Young-Lan Kwak, Jae Hang Shim, Young Suk Shon, and Jae-Kwang Shim. 2022. "Influence of Mild Thyroid Dysfunction on Outcomes after Off-Pump Coronary Artery Bypass Surgery" Journal of Clinical Medicine 11, no. 17: 5033. https://doi.org/10.3390/jcm11175033






