Influence of Radiotherapy on Ossification of Vascularized Osseous Reconstruction of the Jaw: A Radiological Retrospective Cohort Study Based on Panoramic Radiographs
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Data Analysis
3. Results
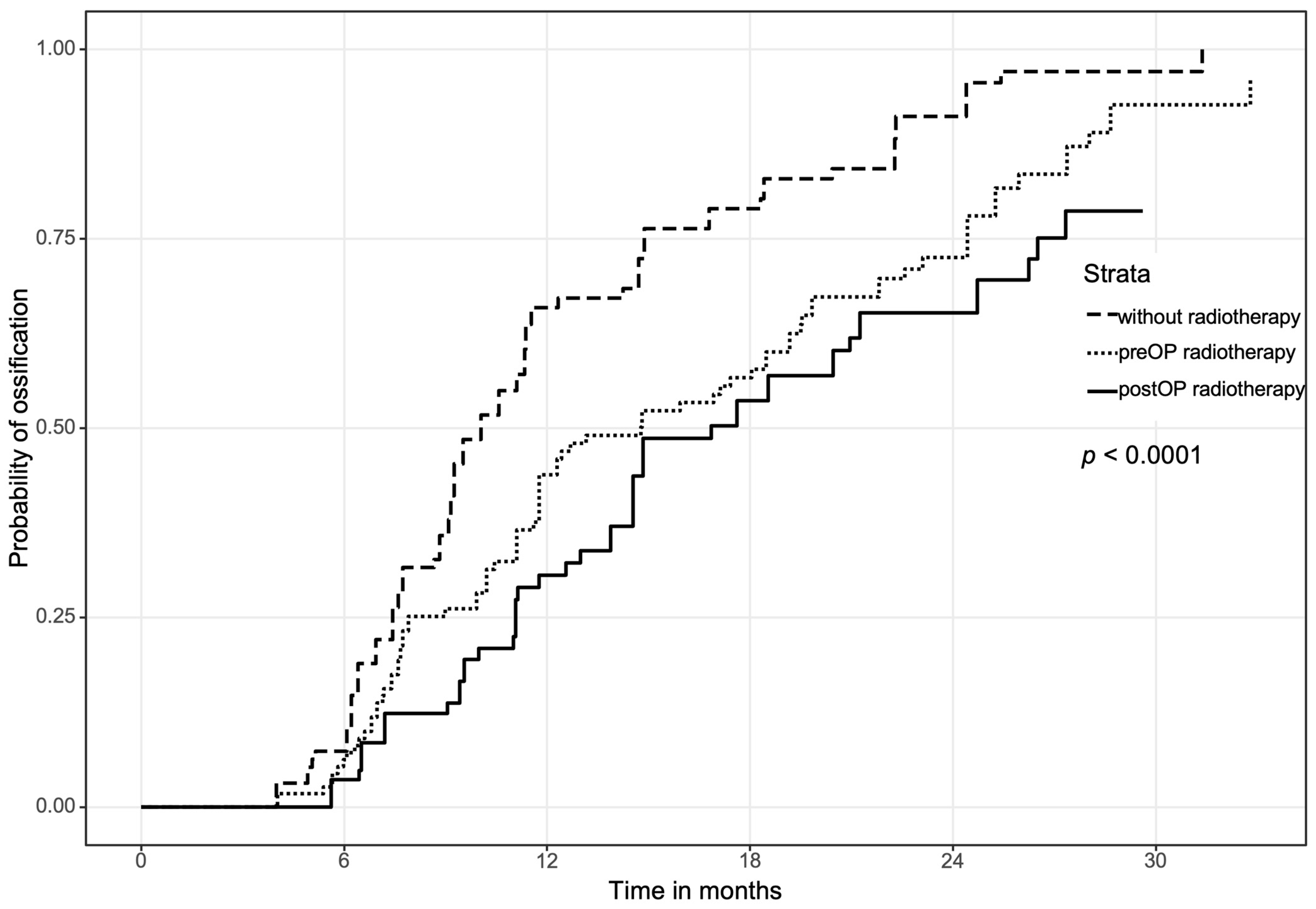
3.1. Overall Ossification between Pre- and Postoperative Radiation Therapy Compared to the Control Group
3.2. Ossification Concerning the Bone Quality
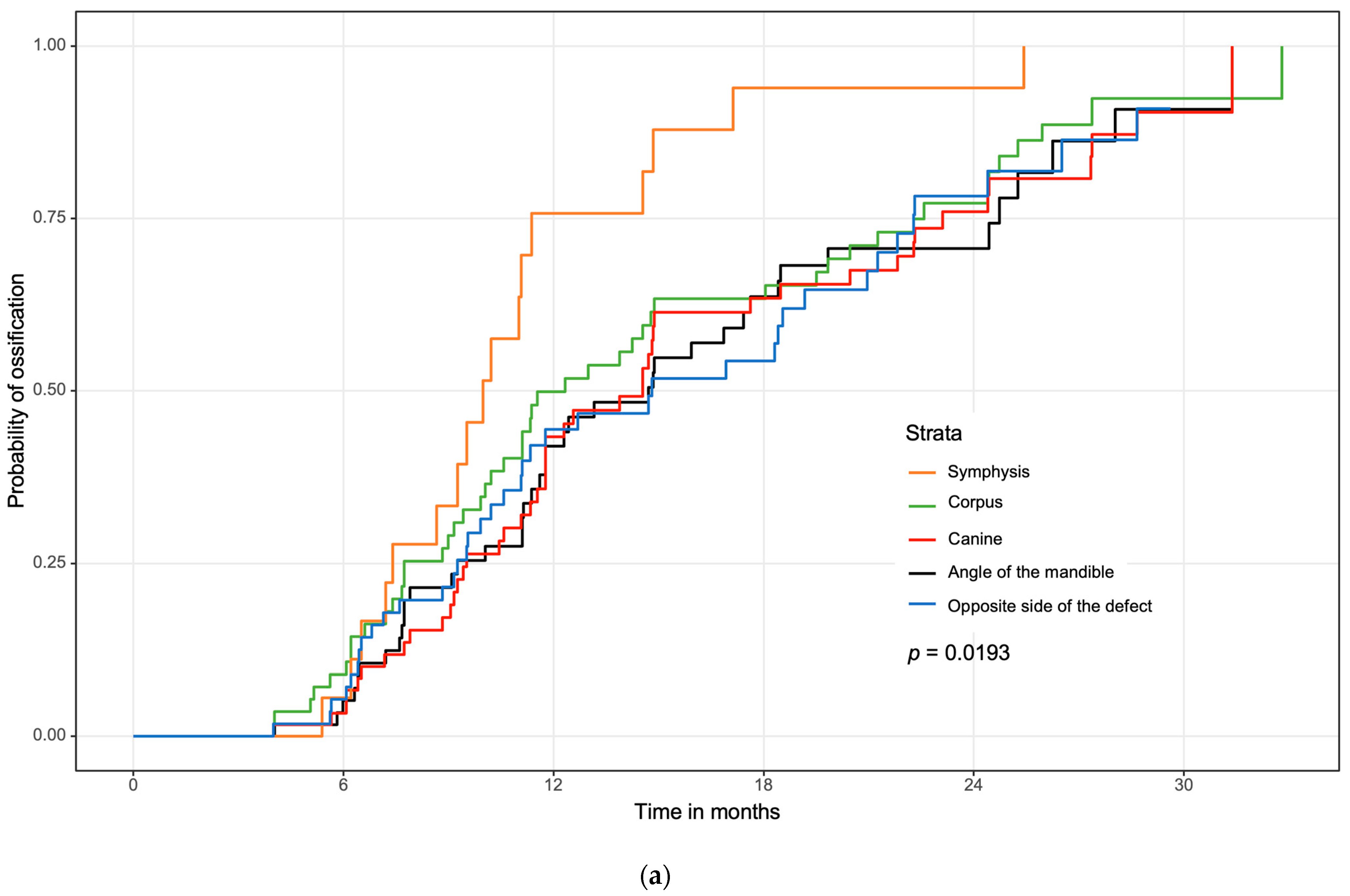
3.3. Regional Ossification
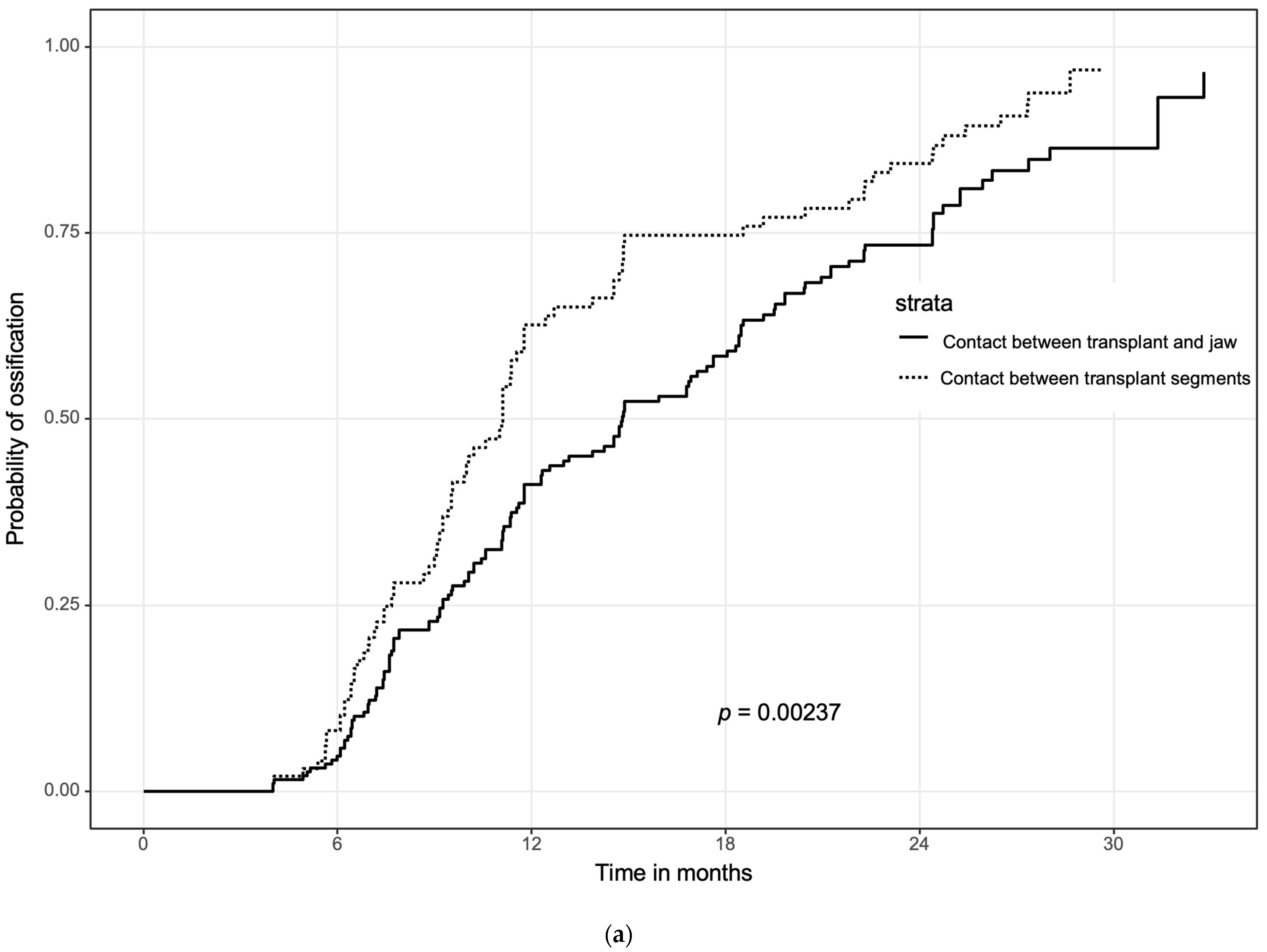
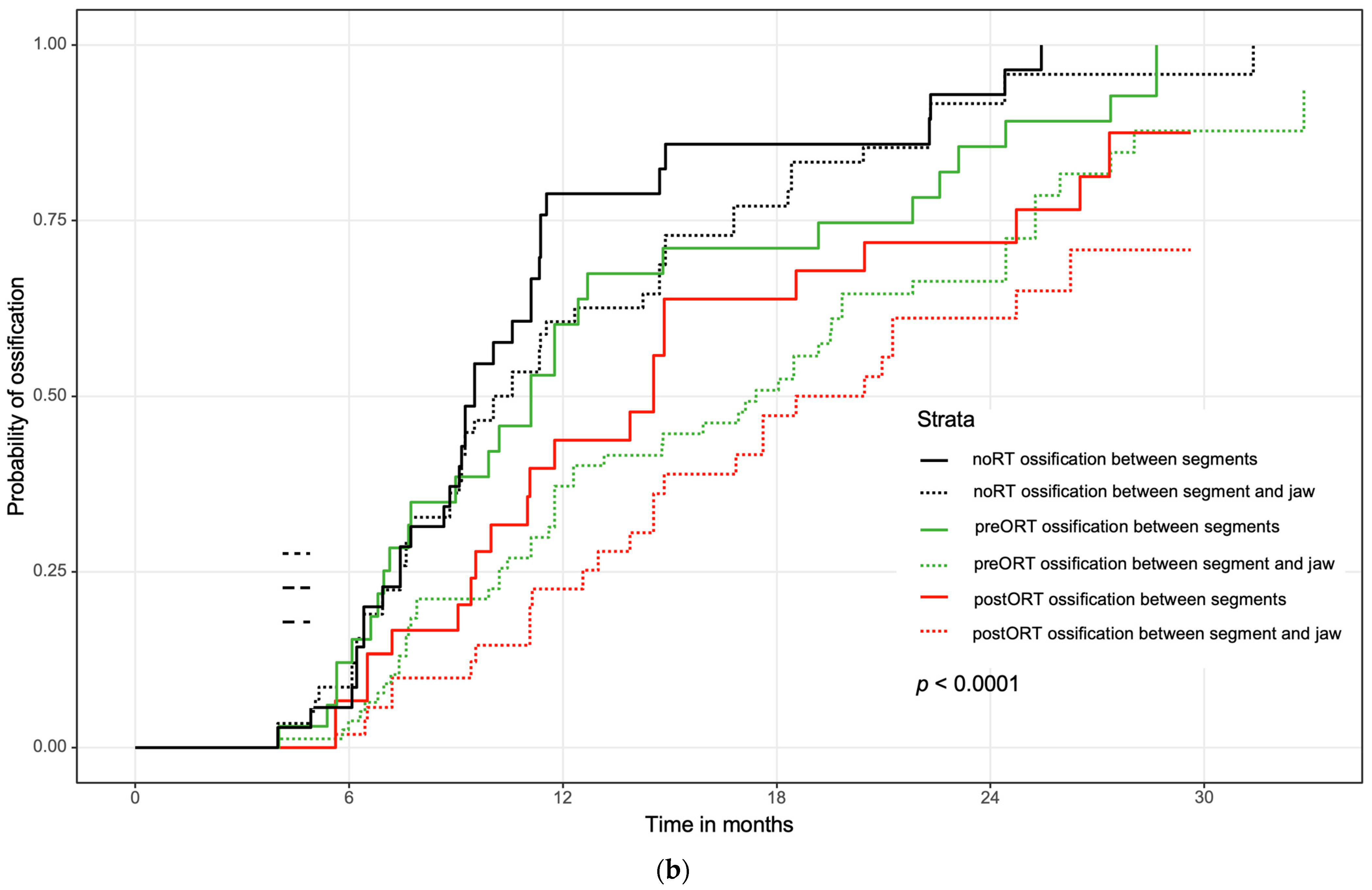
3.4. Influence of Initial Contact between the Bone Segments on Ossification and Analysis of Multiple Variables Regarding Ossification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cannady, S.B.; Lamarre, E.; Wax, M.K. Microvascular Reconstruction: Evidence-Based Procedures. Facial Plast. Surg. Clin. N. Am. 2015, 23, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.D.; Garvey, P.B. Updates in Head and Neck Reconstruction. Plast. Reconstr. Surg. 2018, 141, 271e–285e. [Google Scholar] [CrossRef] [PubMed]
- Al Deek, N.F. Osteoradionecrosis of the mandible: Why not to be more aggressive in earlier stage? Am. J. Otolaryngol. 2020, 41, 102343. [Google Scholar] [CrossRef] [PubMed]
- Haroun, K.; Coblens, O.M. Reconstruction of the mandible for osteoradionecrosis. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 401–406. [Google Scholar] [CrossRef]
- Goh, B.T.; Lee, S.; Tideman, H.; Stoelinga, P.J. Mandibular reconstruction in adults: A review. Int. J. Oral Maxillofac. Surg. 2008, 37, 597–605. [Google Scholar] [CrossRef]
- Vayvada, H.; Mola, F.; Menderes, A.; Yilmaz, M. Surgical Management of Ameloblastoma in the Mandible: Segmental Mandibulectomy and Immediate Reconstruction With Free Fibula or Deep Circumflex Iliac Artery Flap (Evaluation of the Long-Term Esthetic and Functional Results). J. Oral Maxillofac. Surg. 2006, 64, 1532–1539. [Google Scholar] [CrossRef]
- Giudice, A.; Barone, S.; Diodati, F.; Antonelli, A.; Nocini, R.; Cristofaro, M.G. Can Surgical Management Improve Resolution of Medication-Related Osteonecrosis of the Jaw at Early Stages? A Prospective Cohort Study. J. Oral Maxillofac. Surg. 2020, 78, 1986–1999. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.H.; Ahn, K.M. Microvascular reconstruction for maxillofacial defects: A retrospective analysis of outcomes and complications in 121 consecutive cases. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 29. [Google Scholar] [CrossRef]
- Schuderer, J.G.; Meier, J.K.; Klingelhöffer, C.; Gottsauner, M.; Reichert, T.E.; Wendl, C.M.; Ettl, T. Magnetic resonance angiography for free fibula harvest: Anatomy and perforator mapping. Int. J. Oral Maxillofac. Surg. 2020, 49, 176–182. [Google Scholar] [CrossRef]
- Robey, A.B.; Spann, M.L.; McAuliff, T.M.; Meza, J.L.; Hollins, R.R.; Johnson, P.J. Comparison of miniplates and reconstruction plates in fibular flap reconstruction of the mandible. Plast. Reconstr. Surg. 2008, 122, 1733–1738. [Google Scholar] [CrossRef]
- Dean, A.; Alamillos, F.; Heredero, S.; Redondo-Camacho, A.; Guler, I.; Sanjuan, A. Fibula free flap in maxillomandibular reconstruction. Factors related to osteosynthesis plates’ complications. J. Cranio-Maxillofac. Surg. 2020, 48, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Han, H.H.; Kim, H.Y.; Lee, J.Y. The Pros and Cons of Computer-Aided Surgery for Segmental Mandibular Reconstruction after Oncological Surgery. Arch. Craniofac. Surg. 2017, 18, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.Y.; Kim, D.D.; Ghali, G.E. Maxillofacial Reconstruction Using Vascularized Fibula Free Flaps and Endosseous Implants. Oral Maxillofac. Surg. Clin. North Am. 2019, 31, 259–284. [Google Scholar] [CrossRef]
- Navarro Cuéllar, C.; Tousidonis Rial, M.; Antúnez-Conde, R.; Ochandiano Caicoya, S.; Navarro Cuéllar, I.; Arenas de Frutos, G.; Sada Urmeneta, Á.; García-Hidalgo Alonso, M.I.; Navarro Vila, C.; Salmerón Escobar, J.I. Virtual Surgical Planning, Stereolitographic Models and CAD/CAM Titanium Mesh for Three-Dimensional Reconstruction of Fibula Flap with Iliac Crest Graft and Dental Implants. J. Clin. Med. 2021, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Oest, M.E.; Franken, V.; Kuchera, T.; Strauss, J.; Damron, T.A. Long-term loss of osteoclasts and unopposed cortical mineral apposition following limited field irradiation. J. Orthop. Res. 2015, 33, 334–342. [Google Scholar] [CrossRef]
- Jegoux, F.; Malard, O.; Goyenvalle, E.; Aguado, E.; Daculsi, G. Radiation effects on bone healing and reconstruction: Interpretation of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 109, 173–184. [Google Scholar] [CrossRef]
- Mandair, G.S.; Oest, M.E.; Mann, K.A.; Morris, M.D.; Damron, T.A.; Kohn, D.H. Radiation-induced changes to bone composition extend beyond periosteal bone. Bone Rep. 2020, 12, 100262. [Google Scholar] [CrossRef]
- Smith Nobrega, A.; Santiago, J.F.; de Faria Almeida, D.A.; dos Santos, D.M.; Pellizzer, E.P.; Goiato, M.C. Irradiated patients and survival rate of dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2016, 116, 858–866. [Google Scholar] [CrossRef]
- Wolf, F.; Spoerl, S.; Gottsauner, M.; Klingelhöffer, C.; Spanier, G.; Kolbeck, C.; Reichert, T.E.; Hautmann, M.G.; Ettl, T. Significance of site-specific radiation dose and technique for success of implant-based prosthetic rehabilitation in irradiated head and neck cancer patients-A cohort study. Clin. Implant Dent. Relat. Res. 2021, 23, 444–455. [Google Scholar] [CrossRef]
- Ernst, N.; Sachse, C.; Raguse, J.D.; Stromberger, C.; Nelson, K.; Nahles, S. Changes in Peri-Implant Bone Level and Effect of Potential Influential Factors on Dental Implants in Irradiated and Nonirradiated Patients Following Multimodal Therapy Due to Head and Neck Cancer: A Retrospective Study. J. Oral Maxillofac. Surg. 2016, 74, 1965–1973. [Google Scholar] [CrossRef]
- Suh, J.D.; Blackwell, K.E.; Sercarz, J.A.; Cohen, M.; Liu, J.H.; Tang, C.G.; Abemayor, E.; Nabili, V. Disease relapse after segmental resection and free flap reconstruction for mandibular osteoradionecrosis. Otolaryngol. Head Neck Surg. 2010, 142, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Jewer, D.D.; Boyd, J.B.; Manktelow, R.T.; Zuker, R.M.; Rosen, I.B.; Gullane, P.J.; Rotstein, L.E.; Freeman, J.E. Orofacial and mandibular reconstruction with the iliac crest free flap: A review of 60 cases and a new method of classification. Plast. Reconstr. Surg. 1989, 84, 391–403; discussion 395–404. [Google Scholar] [CrossRef] [PubMed]
- Dalinka, M.K.; Edeiken, J.; Finkelstein, J.B. Complications of radiation therapy: Adult bone. Semin. Roentgenol. 1974, 9, 29–40. [Google Scholar] [CrossRef]
- Evans, H.B.; Brown, S.; Hurst, L.N. The effects of early postoperative radiation on vascularized bone grafts. Ann. Plast. Surg. 1991, 26, 505–510. [Google Scholar] [CrossRef]
- Eisenschenk, A.; Witzel, C.; Lautenbach, M.; Ekkernkamp, A.; Weber, U.; Küntscher, M.V. Impact of radiation therapy on healing and stability of vascularized bone grafts in a dog model. Microsurgery 2006, 26, 412–416. [Google Scholar] [CrossRef]
- Oest, M.E.; Policastro, C.G.; Mann, K.A.; Zimmerman, N.D.; Damron, T.A. Longitudinal Effects of Single Hindlimb Radiation Therapy on Bone Strength and Morphology at Local and Contralateral Sites. J. Bone Miner. Res. 2018, 33, 99–112. [Google Scholar] [CrossRef]
- Kubota, H.; Miyawaki, D.; Mukumoto, N.; Ishihara, T.; Matsumura, M.; Hasegawa, T.; Akashi, M.; Kiyota, N.; Shinomiya, H.; Teshima, M.; et al. Risk factors for osteoradionecrosis of the jaw in patients with head and neck squamous cell carcinoma. Radiat. Oncol. 2021, 16, 1. [Google Scholar] [CrossRef]
- Nabil, S.; Samman, N. Risk factors for osteoradionecrosis after head and neck radiation: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 54–69. [Google Scholar] [CrossRef]
- Hirsch, D.L.; Bell, R.B.; Dierks, E.J.; Potter, J.K.; Potter, B.E. Analysis of microvascular free flaps for reconstruction of advanced mandibular osteoradionecrosis: A retrospective cohort study. J. Oral Maxillofac. Surg. 2008, 66, 2545–2556. [Google Scholar] [CrossRef]
- Lee, M.; Chin, R.Y.; Eslick, G.D.; Sritharan, N.; Paramaesvaran, S. Outcomes of microvascular free flap reconstruction for mandibular osteoradionecrosis: A systematic review. J. Cranio-Maxillofac. Surg. 2015, 43, 2026–2033. [Google Scholar] [CrossRef]
- Mulholland, S.; Boyd, J.B.; McCabe, S.; Gullane, P.; Rotstein, L.; Brown, D.; Yoo, J. Recipient vessels in head and neck microsurgery: Radiation effect and vessel access. Plast. Reconstr. Surg. 1993, 92, 628–632. [Google Scholar] [CrossRef]
- Bengtson, B.P.; Schusterman, M.A.; Baldwin, B.J.; Miller, M.J.; Reece, G.P.; Kroll, S.S.; Robb, G.L.; Goepfert, H. Influence of prior radiotherapy on the development of postoperative complications and success of free tissue transfers in head and neck cancer reconstruction. Am. J. Surg. 1993, 166, 326–330. [Google Scholar] [CrossRef]
- Kiener, J.L.; Hoffman, W.Y.; Mathes, S.J. Influence of radiotherapy on microvascular reconstruction in the head and neck region. Am. J. Surg. 1991, 162, 404–407. [Google Scholar] [CrossRef]
- Momeni, A.; Kim, R.Y.; Kattan, A.; Tennefoss, J.; Lee, T.H.C.; Lee, G.K. The effect of preoperative radiotherapy on complication rate after microsurgical head and neck reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Schwartz, D.L.; Farwell, D.G.; Austin-Seymour, M.; Futran, N. Radiation therapy does not impact local complication rates after free flap reconstruction for head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 1308–1312. [Google Scholar] [CrossRef]
- Halle, M.; Bodin, I.; Tornvall, P.; Wickman, M.; Farnebo, F.; Arnander, C. Timing of radiotherapy in head and neck free flap reconstruction--a study of postoperative complications. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 889–895. [Google Scholar] [CrossRef]
- Paderno, A.; Piazza, C.; Bresciani, L.; Vella, R.; Nicolai, P. Microvascular head and neck reconstruction after (chemo)radiation: Facts and prejudices. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 83–90. [Google Scholar] [CrossRef]
- Thankappan, K. Microvascular free tissue transfer after prior radiotherapy in head and neck reconstruction-a review. Surg. Oncol. 2010, 19, 227–234. [Google Scholar] [CrossRef]
- Gordin, E.A.; Ducic, Y. Microvascular free tissue reconstruction in the patient with multiple courses of radiation. Laryngoscope 2014, 124, 2252–2256. [Google Scholar] [CrossRef]
- Palma, L.F.; Tateno, R.Y. Impact of radiotherapy on mandibular bone: A retrospective study of digital panoramic radiographs. Imaging Sci. Dent. 2020, 50, 31–36. [Google Scholar] [CrossRef]
- Swendseid, B.; Kumar, A.; Sweeny, L.; Zhan, T.; Goldman, R.A.; Krein, H.; Heffelfinger, R.N.; Luginbuhl, A.J.; Curry, J.M. Natural History and Consequences of Nonunion in Mandibular and Maxillary Free Flaps. Otolaryngol. Head Neck Surg. 2020, 163, 956–962. [Google Scholar] [CrossRef] [PubMed]
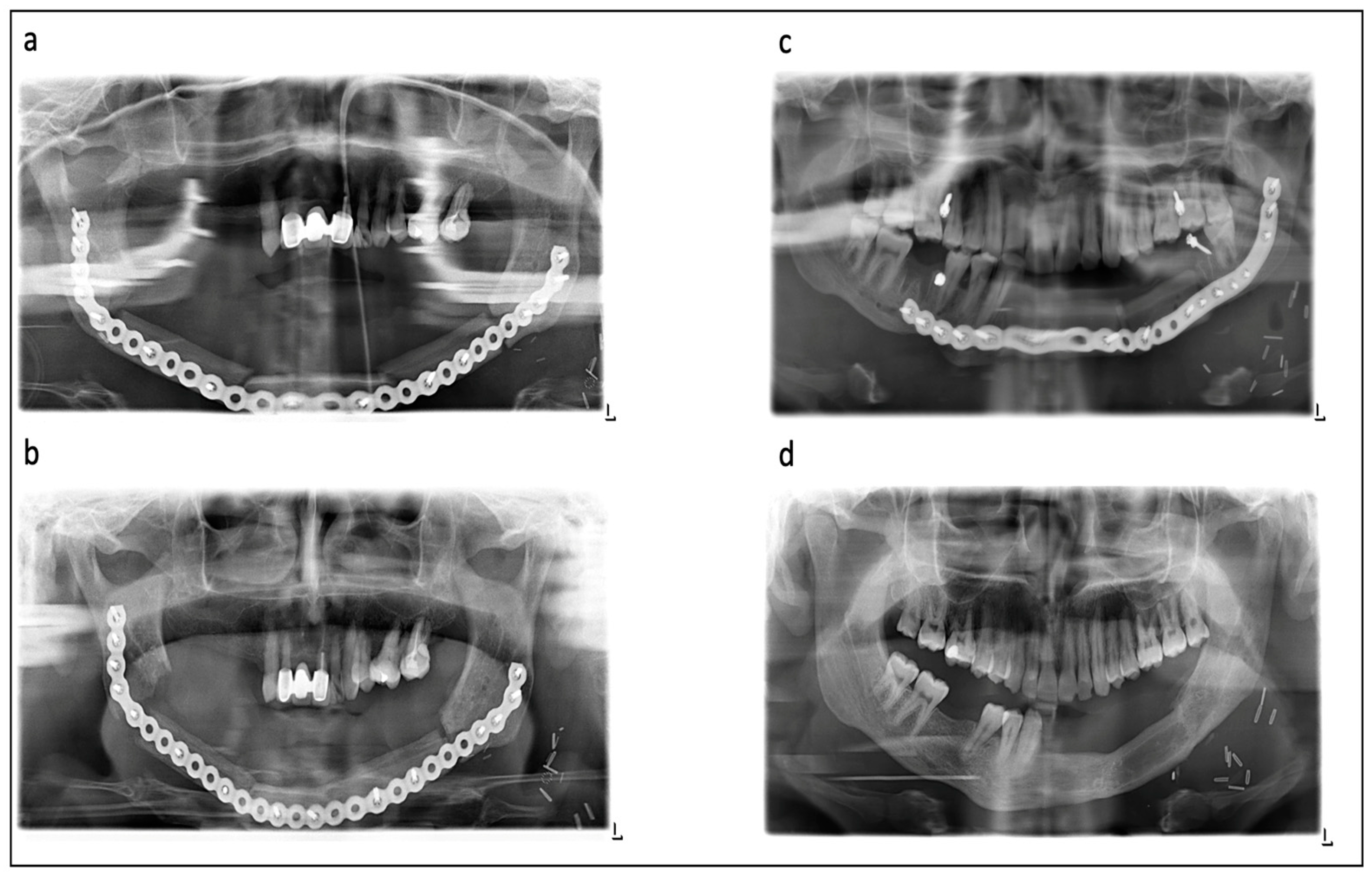

| Postoperative Radiotherapy (n = 26) | Preoperative Radiotherapy (n = 41) | Nonirradiated (Control Group) (n = 33) | |
|---|---|---|---|
| (a) | |||
| Male/female, n (%) | 20 (77%)/6 (23%) | 26 (63%)/15 (37%) | 9 (58%)/14 (42%) |
| Age in years, median (IQR) | 59 (51, 64) | 59 (51, 67) | 53 (44, 63) |
| Radiation dose in Gy, median (IQR) | 64.9 (60.0, 66.6) | 66.0 (60.0, 70.0) | none |
| Tobacco, n (%) | 20 (77%) | 27 (66%) | 15 (46%) |
| CAD/CAM planning, n (%) | 0 (0%) | 7 (17%) | 4 (12%) |
| Neck dissection, n (%) | 25 (96%) | 25 (61%) | 11 (33%) |
| (b) | |||
| Primary Diagnosis | |||
| Malignant Tumor | 26 | 18 | 16 |
| Osteoradionecrosis | 0 | 23 | 0 |
| MRONJ | 0 | 0 | 2 |
| Unspecific Osteomyelitis | 0 | 0 | 10 |
| Gun shot wounds | 0 | 0 | 3 |
| Benigne Tumor | 0 | 0 | 2 |
| Side of malignancy (n = 62) | |||
| Lateral Floor of Mouth/Mandibel | 25 | 16 | 14 |
| Maxilla | 0 | 1 | 5 |
| Skin | 0 | 1 | 0 |
| Side of primary malignancy in ORN (n = 23) | |||
| Lateral Floor of Mouth/Mandibel | 5 | ||
| Anterior Floor of Mouth/Lower Lip | 1 | ||
| Tongue | 2 | ||
| Pharynx/Tonsille/Root of Tongue | 9 | ||
| Larynx | 1 | ||
| Maxilla/Nose/Sinus maxillaris | 2 | ||
| Parotid Gland | 1 | ||
| More than one Location | 2 | ||
| (c) | |||
| Defect Classification of Boyd and Jewer | |||
| C | 1 | 0 | 1 |
| H | 0 | 1 | 1 |
| HC | 0 | 0 | 2 |
| HCL | 0 | 0 | 1 |
| HL | 0 | 1 | 0 |
| L | 7 | 19 | 6 |
| LC | 5 | 7 | 9 |
| LCL | 13 | 12 | 8 |
| Maxilla | 0 | 1 | 5 |
| Typ of flaps | |||
| Fibula | 24 | 36 | 26 |
| Iliac crest | 1 | 5 | 5 |
| Scapula | 1 | 0 | 2 |
| postORT and Contact between Graft and Original Bone n = 53 | preORT and Contact between Graft and Original Bone n = 82 | noRT and Contact between Graft and Original Bone n = 62 | postORT and Contact between Graft Segments n = 30 | preORT and Contact between Graft Segments n = 33 | noRT and Contact between Graft Segments n = 35 | |
|---|---|---|---|---|---|---|
| postORT and contact between graft and original bone | 0.164 | <0.0001 | 0.094 | 0.001 | <0.0001 | |
| preORT and contact between graft and original bone | 0.164 | <0.0001 | 0.601 | 0.015 | <0.0001 | |
| noRT and contact between graft and original bone | <0.0001 | <0.0001 | 0.040 | 0.659 | 0.334 | |
| postORT and contact between graft segments | 0.094 | 0.601 | 0.040 | 0.135 | 0.003 | |
| preORT and contact between graft segments | 0.001 | 0.015 | 0.659 | 0.135 | 0.128 | |
| noRT and contact between graft segments | <0.0001 | <0.0001 | 0.334 | 0.003 | 0.128 |
| HR | 95%-CI | p-Value | ||
|---|---|---|---|---|
| Radiotherapy | ||||
| Reference | |||
| 0.529 | 0.353 | 0.792 | 0.002 |
| 0.728 | 0.514 | 1.031 | 0.074 |
| No tabacco abuse | 1.350 | 0.980 | 1.860 | 0.066 |
| Contact between transplant segments | 1.391 | 1.024 | 1.890 | 0.035 |
| Amount of segments of the transplant | ||||
| Reference | |||
| 1.685 | 1.113 | 2.552 | 0.014 |
| 1.290 | 0.820 | 2.031 | 0.271 |
| Quality of initial contact | ||||
| Reference | |||
| 1.055 | 0.473 | 2.351 | 0.896 |
| 1.523 | 0.705 | 3.292 | 0.285 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottsauner, M.; Fehrer, C.; Spoerl, S.; Schuderer, J.; Zeman, F.; Fiedler, M.; Maurer, M.; Reichert, T.E.; Ettl, T. Influence of Radiotherapy on Ossification of Vascularized Osseous Reconstruction of the Jaw: A Radiological Retrospective Cohort Study Based on Panoramic Radiographs. J. Clin. Med. 2022, 11, 5041. https://doi.org/10.3390/jcm11175041
Gottsauner M, Fehrer C, Spoerl S, Schuderer J, Zeman F, Fiedler M, Maurer M, Reichert TE, Ettl T. Influence of Radiotherapy on Ossification of Vascularized Osseous Reconstruction of the Jaw: A Radiological Retrospective Cohort Study Based on Panoramic Radiographs. Journal of Clinical Medicine. 2022; 11(17):5041. https://doi.org/10.3390/jcm11175041
Chicago/Turabian StyleGottsauner, Maximilian, Clara Fehrer, Steffen Spoerl, Johannes Schuderer, Florian Zeman, Mathias Fiedler, Michael Maurer, Torsten E. Reichert, and Tobias Ettl. 2022. "Influence of Radiotherapy on Ossification of Vascularized Osseous Reconstruction of the Jaw: A Radiological Retrospective Cohort Study Based on Panoramic Radiographs" Journal of Clinical Medicine 11, no. 17: 5041. https://doi.org/10.3390/jcm11175041
APA StyleGottsauner, M., Fehrer, C., Spoerl, S., Schuderer, J., Zeman, F., Fiedler, M., Maurer, M., Reichert, T. E., & Ettl, T. (2022). Influence of Radiotherapy on Ossification of Vascularized Osseous Reconstruction of the Jaw: A Radiological Retrospective Cohort Study Based on Panoramic Radiographs. Journal of Clinical Medicine, 11(17), 5041. https://doi.org/10.3390/jcm11175041








