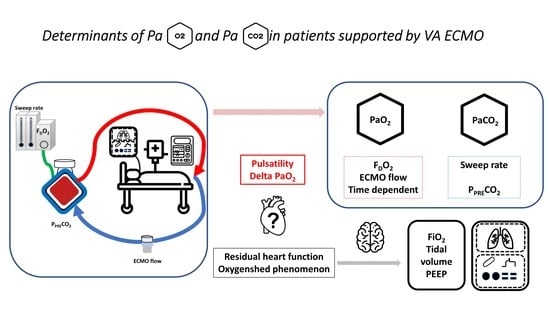
Determinants of Arterial Pressure of Oxygen and Carbon Dioxide in Patients Supported by Veno-Arterial ECMO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients’ Management
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. Determinants of Arterial Partial Pressure of Oxygen (PaO2)
3.2. Determinants of Arterial Partial Pressure of Dioxide Carbon (PaCO2)
3.3. Determinants of Delta-PaO2 (Difference between Post-Membrane PO2 and PaO2)
3.4. Accordance with ELSO Guidelines
4. Discussion
4.1. Clinical Implications
4.2. Limits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamm, C.W.; Bassand, J.-P.; Agewall, S.; Bax, J.; Boersma, E.; Bueno, H.; Caso, P.; Dudek, D.; Gielen, S.; Huber, K.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar] [CrossRef]
- Wong, A.S.K.; Sin, S.W.C. Short-term mechanical circulatory support (intra-aortic balloon pump, Impella, extracorporeal membrane oxygenation, TandemHeart): A review. Ann. Transl. Med. 2020, 8, 829. [Google Scholar] [CrossRef]
- Welker, C.; Huang, J.; Ramakrishna, H. Analysis of the 2020 EACTS/ELSO/STS/AATS Expert Guidelines on the Management of Adult Postcardiotomy Extracorporeal Life Support. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2207–2219. [Google Scholar] [CrossRef]
- Tsangaris, A.; Alexy, T.; Kalra, R.; Kosmopoulos, M.; Elliott, A.; Bartos, J.A.; Yannopoulos, D. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support for the Management of Cardiogenic Shock. Front. Cardiovasc. Med. 2021, 8, 686558. [Google Scholar] [CrossRef]
- Koeltz, A.; Gendron, N.; Ajzenberg, N.; Longrois, D. How to Manage Thrombocytopenia with ECLS: A Proposal of Clinical Reasoning Tools. J. Extra-Corpor. Technol. 2018, 50, 256–259. [Google Scholar]
- Geyer, M.; Gohrbandt, B.; Sagoschen, I.; Hartmann, T.; Post, F.; Vahl, C.-F.; Münzel, T. Pitfalls of cannulation for extracorporeal life support: Review of the literature and illustrative case presentation. J. Artif. Organs 2018, 21, 8–16. [Google Scholar] [CrossRef]
- Guimaron, S.; Laverdure, F.; Andrei, S.; Kortchinsky, T.; Thès, J.; Stéphan, F. Reimplantation of Venoarterial Extracorporeal Membrane Oxygenation (ECMO) After Withdrawal Failure. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2360–2361. [Google Scholar] [CrossRef]
- Andrei, S.; Nguyen, M.; Berthoud, V.; Morgant, M.-C.; Bouhemad, B.; Guinot, P.-G.; ECMORIX Study Group. Evaluation of the Oxiris Membrane in Cardiogenic Shock Requiring Extracorporeal Membrane Oxygenation Support: Study Protocol for a Single Center, Single-Blind, Randomized Controlled Trial. Front. Cardiovasc. Med. 2021, 8, 738496. [Google Scholar] [CrossRef]
- Chung, M.; Shiloh, A.L.; Carlese, A. Monitoring of the adult patient on venoarterial extracorporeal membrane oxygenation. Sci. World J. 2014, 2014, 393258. [Google Scholar] [CrossRef]
- Gu, K.; Zhang, Z.; Gao, B.; Chang, Y.; Wan, F. Hemodynamic effects of perfusion level of peripheral ECMO on cardiovascular system. Biomed. Eng. OnLine 2018, 17, 59. [Google Scholar] [CrossRef]
- Schmidt, M.; Tachon, G.; Devilliers, C.; Muller, G.; Hekimian, G.; Bréchot, N.; Merceron, S.; Luyt, C.E.; Trouillet, J.-L.; Chastre, J.; et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensiv. Care Med. 2013, 39, 838–846. [Google Scholar] [CrossRef]
- Messai, E.; Bouguerra, A.; Harmelin, G.; Di Lascio, G.; Bonizzoli, M.; Bonacchi, M. A numerical model of blood oxygenation during veno-venous ECMO: Analysis of the interplay between blood oxygenation and its delivery parameters. J. Clin. Monit. Comput. 2016, 30, 327–332. [Google Scholar] [CrossRef]
- Park, M.; Costa, E.L.V.; Maciel, A.T.; Silva, D.P.E.; Friedrich, N.; Barbosa, E.V.S.; Hirota, A.S.; Schettino, G.; Azevedo, L.C.P. Determinants of oxygen and carbon dioxide transfer during extracorporeal membrane oxygenation in an experimental model of multiple organ dysfunction syndrome. PLoS ONE 2013, 8, e54954. [Google Scholar] [CrossRef]
- Spinelli, E.; Colussi, G.; Dal Santo, G.; Scotti, E.; Marongiu, I.; Garbelli, E.; Mazzucco, A.; Dondossola, D.; Maia, R.; Battistin, M.; et al. Atelectasis, Shunt, and Worsening Oxygenation Following Reduction of Respiratory Rate in Healthy Pigs Undergoing ECMO: An Experimental Lung Imaging Study. Front. Physiol. 2021, 12, 663313. [Google Scholar] [CrossRef]
- Lorusso, R.; Shekar, K.; MacLaren, G.; Schmidt, M.; Pellegrino, V.; Meyns, B.; Haft, J.; Vercaemst, L.; Pappalardo, F.; Bermudez, C.; et al. ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. ASAIO J. 2021, 67, 827–844. [Google Scholar] [CrossRef]
- Winiszewski, H.; Guinot, P.-G.; Schmidt, M.; Besch, G.; Piton, G.; Perrotti, A.; Lorusso, R.; Kimmoun, A.; Capellier, G. Optimizing PO2 during peripheral veno-arterial ECMO: A narrative review. Crit. Care 2022, 26, 226. [Google Scholar] [CrossRef]
- Ellouze, O.; Lamirel, J.; Perrot, J.; Missaoui, A.; Daily, T.; Aho, S.; Petrosyan, A.; Guinot, P.G.; Bouchot, O.; Bouhemad, B. Extubation of patients undergoing extracorporeal life support. A retrospective study. Perfusion 2019, 34, 50–57. [Google Scholar] [CrossRef]
- Ellouze, O.; Abbad, X.; Constandache, T.; Missaoui, A.; Berthoud, V.; Daily, T.; Aho, S.; Bouchot, O.; Bouhemad, B.; Guinot, P.-G. Risk Factors of Bleeding in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. Ann. Thorac. Surg. 2021, 111, 623–628. [Google Scholar] [CrossRef]
- Ellouze, O.; Soudry Faure, A.; Radhouani, M.; Abou-Arab, O.; Besnier, E.; Moussa, M.; Cransac, A.; Ksiazek, E.; Fischer, M.-O.; Mertes, P.M.; et al. Levosimendan in venoarterial ECMO weaning. Rational and design of a randomized double blind multicentre trial. ESC Heart Fail. 2021, 8, 3339–3347. [Google Scholar] [CrossRef]
- Ellouze, O.; Nguyen, M.; Missaoui, A.; Berthoud, V.; Aho, S.; Bouchot, O.; Guinot, P.G.; Bouhemad, B. Prognosis Value of Early Veno Arterial PCO2 Difference in Patients Under Peripheral Veno Arterial Extracorporeal Membrane Oxygenation. Shock 2020, 54, 744–750. [Google Scholar] [CrossRef]
- Schmidt, M.; Burrell, A.; Roberts, L.; Bailey, M.; Sheldrake, J.; Rycus, P.T.; Hodgson, C.; Scheinkestel, C.; Cooper, D.J.; Thiagarajan, R.R.; et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur. Heart J. 2015, 36, 2246–2256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Gao, B.; Chang, Y. The numerical study on the effects of cardiac function on the aortic oxygen distribution. Med. Biol. Eng. Comput. 2018, 56, 1305–1313. [Google Scholar] [CrossRef]
- Spinelli, E.; Bartlett, R.H. Relationship between hemoglobin concentration and extracorporeal blood flow as determinants of oxygen delivery during venovenous extracorporeal membrane oxygenation: A mathematical model. ASAIO J. 2014, 60, 688–693. [Google Scholar] [CrossRef]
- Moussa, M.D.; Beyls, C.; Lamer, A.; Roksic, S.; Juthier, F.; Leroy, G.; Petitgand, V.; Rousse, N.; Decoene, C.; Dupré, C.; et al. Early hyperoxia and 28-day mortality in patients on venoarterial ECMO support for refractory cardiogenic shock: A bicenter retrospective propensity score-weighted analysis. Crit. Care 2022, 26, 257. [Google Scholar] [CrossRef]
- Lubnow, M.; Philipp, A.; Foltan, M.; Enger, T.B.; Lunz, D.; Bein, T.; Haneya, A.; Schmid, C.; Riegger, G.; Müller, T.; et al. Technical Complications during Veno-Venous Extracorporeal Membrane Oxygenation and Their Relevance Predicting a System-Exchange—Retrospective Analysis of 265 Cases. PLoS ONE 2014, 9, e112316. [Google Scholar] [CrossRef]
- Qi, J.; Gao, S.; Liu, G.; Yan, S.; Zhang, M.; Yan, W.; Zhang, Q.; Teng, Y.; Wang, J.; Zhou, C.; et al. An Ovine Model of Awake Veno-Arterial Extracorporeal Membrane Oxygenation. Front. Vet. Sci. 2021, 8, 809487. [Google Scholar] [CrossRef]
- Park, M.; Mendes, P.V.; Costa, E.L.V.; Barbosa, E.V.S.; Hirota, A.S.; Azevedo, L.C.P. Factors associated with blood oxygen partial pressure and carbon dioxide partial pressure regulation during respiratory extracorporeal membrane oxygenation support: Data from a swine model. Rev. Bras. Ter. Intensiv. 2016, 28, 11–18. [Google Scholar] [CrossRef]
- Mourad, M.; Eliet, J.; Zeroual, N.; Saour, M.; Sentenac, P.; Manna, F.; Molinari, N.; Gandet, T.; Colson, P.H.; Gaudard, P. Pulse pressure and end-tidal carbon dioxide for monitoring low native cardiac output during veno-arterial ECLS: A prospective observational study. Crit. Care 2020, 24, 569. [Google Scholar] [CrossRef]
- Rupprecht, L.; Lunz, D.; Philipp, A.; Lubnow, M.; Schmid, C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel. 2015, 7, 320–326. [Google Scholar]
- Ghalayini, M.; Brun, P.-Y.; Augustin, P.; Guivarch, E.; Dilly, M.P.; Provenchere, S.; Mordant, P.; Castier, Y.; Montravers, P.; Longrois, D. Esmolol Corrects Severe Hypoxemia in Patients with Femoro-Femoral Venoarterial Extracorporeal Life Support for Lung Transplantation. J. Extra-Corpor. Technol. 2016, 48, 113–121. [Google Scholar]
- Andrei, S.; Tran-Dinh, A.; Provenchere, S.; Lortat-Jacob, B.; Ghodbane, W.; Montravers, P.; Longrois, D. A quantified description of the interactions between the native cardiovascular system and femoro-femoral versus femoro-axillary extracorporeal life support using descending thoracic aorta velocity time integral. Artif. Organs 2019, 43, 647–655. [Google Scholar] [CrossRef]
- Stevens, M.C.; Callaghan, F.M.; Forrest, P.; Bannon, P.G.; Grieve, S.M. Flow mixing during peripheral veno-arterial extra corporeal membrane oxygenation—A simulation study. J. Biomech. 2017, 55, 64–70. [Google Scholar] [CrossRef]
- Avgerinos, D.V.; DeBois, W.; Voevidko, L.; Salemi, A. Regional variation in arterial saturation and oxygen delivery during venoarterial extracorporeal membrane oxygenation. J. Extra-Corpor. Technol. 2013, 45, 183–186. [Google Scholar]
- Justus, A.; Burrell, A.; Anstey, C.; Cornmell, G.; Brodie, D.; Shekar, K. The Association of Oxygenation, Carbon Dioxide Removal, and Mechanical Ventilation Practices on Survival During Venoarterial Extracorporeal Membrane Oxygenation. Front. Med. 2021, 8, 756280. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Munshi, L.; Del Sorbo, L.; Fan, E. The Early Change in PaCO2 after Extracorporeal Membrane Oxygenation Initiation Is Associated with Neurological Complications. Am. J. Respir. Crit. Care Med. 2020, 201, 1525–1535. [Google Scholar] [CrossRef]
- Amado-Rodríguez, L.; Del Busto, C.; López-Alonso, I.; Parra, D.; Mayordomo-Colunga, J.; Arias-Guillén, M.; Albillos-Almaraz, R.; Martín-Vicente, P.; López-Martínez, C.; Huidobro, C.; et al. Biotrauma during ultra-low tidal volume ventilation and venoarterial extracorporeal membrane oxygenation in cardiogenic shock: A randomized crossover clinical trial. Ann. Intensiv. Care 2021, 11, 132. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Stremmel, C.; Stark, K.; Joskowiak, D.; Czermak, T.; Born, F.; Kupka, D.; Scherer, C.; Orban, M.; Petzold, T.; et al. Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. J. Clin. Med. 2020, 9, 992. [Google Scholar] [CrossRef]
- Chiu, L.-C.; Kao, K.-C. Mechanical Ventilation during Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome: A Narrative Review. J. Clin. Med. 2021, 10, 4953. [Google Scholar] [CrossRef]
- Chang, W.-T.; Wang, C.-H.; Lai, C.-H.; Yu, H.-Y.; Chou, N.-K.; Wang, C.-H.; Huang, S.-C.; Tsai, P.-R.; Chou, F.-J.; Tsai, M.-S.; et al. Optimal Arterial Blood Oxygen Tension in the Early Postresuscitation Phase of Extracorporeal Cardiopulmonary Resuscitation: A 15-Year Retrospective Observational Study. Crit. Care Med. 2019, 47, 1549–1556. [Google Scholar] [CrossRef]
- Al-Kawaz, M.N.; Canner, J.; Caturegli, G.; Kannapadi, N.; Balucani, C.; Shelley, L.; Kim, B.S.; Choi, C.W.; Geocadin, R.G.; Whitman, G.; et al. Duration of Hyperoxia and Neurologic Outcomes in Patients Undergoing Extracorporeal Membrane Oxygenation. Crit. Care Med. 2021, 49, e968–e977. [Google Scholar] [CrossRef]
| Variables | All Cohort (n = 45) |
|---|---|
| Age (years), mean ± SD | 58 ± 11 |
| Female gender, n (%) | 10 (22) |
| BMI (kg m−2), mean ± SD | 28.2 ± 5 |
| SOFA score, mean ± SD | 13 ± 2 |
| SAPS 2 score, mean ± SD | 69 ± 22 |
| Pulsatility (yes), n (%) | 21 (47) |
| Vasoactive and inotropic agents | |
| Norepinephrine, n (%) | 29 (64) |
| Dobutamine, n (%) | 12 (27) |
| Epinephrine, n (%) | 26 (58) |
| Indication for VA-ECMO, n (%) | |
| Cardiac Arrest | 12 (27) |
| Post cardiotomy shock | 15 (33) |
| Medical cardiogenic shock | 15 (34) |
| Drug intoxication | 3 (7) |
| ECMO baseline parameters | |
| 4.1 [3.7–4.8] |
| 4.5 [4–5.4] |
| 80 [70–100] |
| Ventilatory parameters | |
| 60 [50–100] |
| 14 [12–16] |
| 5.8 [5.1–6.4] |
| 5 [5,6] |
| 28-day mortality, n (%) | 28 (62) |
| Variables | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| Estimate | Std Error | p-Value | Estimate | Std Error | p-Value | |
| Norepinephrine (yes) | −20.2 | 18.1 | 0.267 | |||
| Dobutamine (yes) | −23 | 18.5 | 0.216 | |||
| Epinephrine (yes) | 21 | 19 | 0.273 | |||
| Pulsatility (yes) | −6.3 | 16.6 | 0.702 | |||
| Hemoglobin (g dL−1) | −6.5 | 4 | 0.112 | |||
| Ventilatory parameters | ||||||
| FiO2 (%) | 0.99 | 0.47 | 0.037 | 0.33 | 0.44 | 0.442 |
| Minute volume (mL kg−1) | 0.21 | 0.42 | 0.614 | |||
| PEEP (cmH20) | −6 | 4.2 | 0.151 | |||
| ECMO parameters | ||||||
| QEC | −23.8 | 10.2 | 0.020 | −23.6 | 9.87 | 0.018 |
| % of theoretical flow | −0.97 | 0.45 | 0.03 | |||
| Sweep rate | 14.5 | 5.4 | 0.007 | 5.11 | 5.44 | 0.348 |
| FDO2 | 3.1 | 0.47 | <0.001 | 2.33 | 0.52 | <0.001 |
| Pre-membrane blood gases | ||||||
| pH | −224 | 59 | <0.001 | −238 | 160 | 0.139 |
| PO2 | 0.26 | 0.23 | 0.267 | |||
| PCO2 | −0.05 | 1 | 0.957 | |||
| HCO3− | −0.37 | 0.6 | 0.539 | |||
| Blood Saturation | 1.1 | 0.54 | 0.04 | 0.89 | 0.49 | 0.069 |
| Post-membrane blood gases | ||||||
| pH | −188 | 62 | 0.002 | 45.4 | 165 | 0.784 |
| PO2 | 0.56 | 0.06 | <0.001 | |||
| PCO2 | −1.3 | 1.09 | 0.245 | |||
| HCO3− | −5.4 | 1.8 | 0.003 | 1.19 | 2.19 | 0.588 |
| Blood saturation (%) | 1.8 | 1.07 | 0.093 | |||
| Time point of measurement | −20.5 | 7.6 | 0.007 | |||
| Variables | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| Estimate | Std Error | p-Value | Estimate | Std Error | p-Value | |
| Norepinephrine (yes) | −0.19 | 1.37 | 0.892 | |||
| Dobutamine (yes) | −1.89 | 1.42 | 0.185 | |||
| Epinephrine (yes) | 1.71 | 1.43 | 0.237 | |||
| Pulsatility (yes) | −2.13 | 1.24 | 0.087 | |||
| Hemoglobin (g dL−1) | −0.22 | 0.3 | 0.45 | |||
| Ventilatory parameters | ||||||
| FiO2 (%) | −0.06 | 0.03 | 0.11 | |||
| Minute volume (mL kg−1) | 0.002 | 0.02 | 0.93 | |||
| PEEP (cmH20) | 0.01 | 0.31 | 0.966 | |||
| VA ECMO parameters | ||||||
| QEC | 0.61 | 0.78 | 0.439 | |||
| Sweep rate flow | 0.5 | 0.41 | 0.227 | 0.66 | 0.28 | 0.022 |
| FDO2 | 1.45 | 0.04 | 0.833 | |||
| Pre-membrane blood gases | ||||||
| pH | −15 | 4.5 | 0.001 | 9.27 | 9.3 | 0.322 |
| PO2 | −0.008 | 0.01 | 0.63 | |||
| PCO2 | 0.66 | 0.05 | <0.001 | 0.72 | 0.06 | <0.001 |
| HCO3− | 0.005 | 0.04 | 0.895 | |||
| Blood saturation | −0.09 | 0.04 | 0.03 | 0.06 | 0.03 | 0.043 |
| Post-membrane blood gases | ||||||
| pH | −16.7 | 4.6 | <0.001 | −5 | 9.44 | 0.597 |
| PO2 | −0.01 | 0.005 | 0.008 | −0.006 | 0.003 | 0.078 |
| PCO2 | 0.72 | 0.057 | <0.001 | |||
| HCO3− | 0.17 | 0.14 | 0.224 | |||
| Blood saturation | −0.04 | 0.08 | 0.552 | |||
| Time point of measurement | −0.3 | 0.4 | 0.448 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrei, S.; Nguyen, M.; Berthoud, V.; Durand, B.; Duclos, V.; Morgant, M.-C.; Bouchot, O.; Bouhemad, B.; Guinot, P.-G. Determinants of Arterial Pressure of Oxygen and Carbon Dioxide in Patients Supported by Veno-Arterial ECMO. J. Clin. Med. 2022, 11, 5228. https://doi.org/10.3390/jcm11175228
Andrei S, Nguyen M, Berthoud V, Durand B, Duclos V, Morgant M-C, Bouchot O, Bouhemad B, Guinot P-G. Determinants of Arterial Pressure of Oxygen and Carbon Dioxide in Patients Supported by Veno-Arterial ECMO. Journal of Clinical Medicine. 2022; 11(17):5228. https://doi.org/10.3390/jcm11175228
Chicago/Turabian StyleAndrei, Stefan, Maxime Nguyen, Vivien Berthoud, Bastian Durand, Valerian Duclos, Marie-Catherine Morgant, Olivier Bouchot, Belaid Bouhemad, and Pierre-Grégoire Guinot. 2022. "Determinants of Arterial Pressure of Oxygen and Carbon Dioxide in Patients Supported by Veno-Arterial ECMO" Journal of Clinical Medicine 11, no. 17: 5228. https://doi.org/10.3390/jcm11175228
APA StyleAndrei, S., Nguyen, M., Berthoud, V., Durand, B., Duclos, V., Morgant, M.-C., Bouchot, O., Bouhemad, B., & Guinot, P.-G. (2022). Determinants of Arterial Pressure of Oxygen and Carbon Dioxide in Patients Supported by Veno-Arterial ECMO. Journal of Clinical Medicine, 11(17), 5228. https://doi.org/10.3390/jcm11175228