Three-Dimensional Printing and Fracture Mapping in Pelvic and Acetabular Fractures: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Study Identification
2.4. Data Extraction
2.5. Study Characteristics
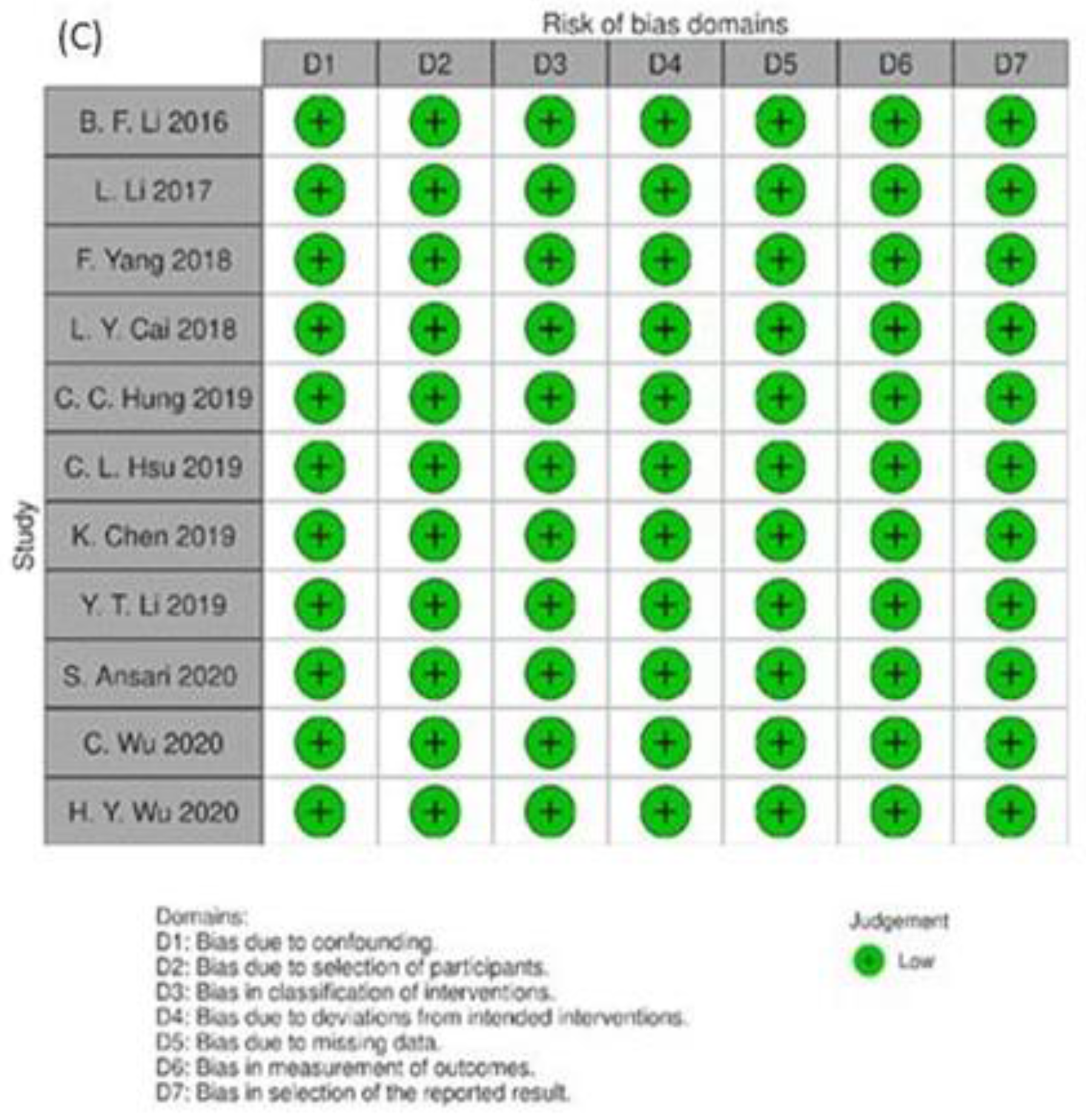
2.6. Risk of Bias Assessment
2.7. Data Synthesis and Statistical Analysis
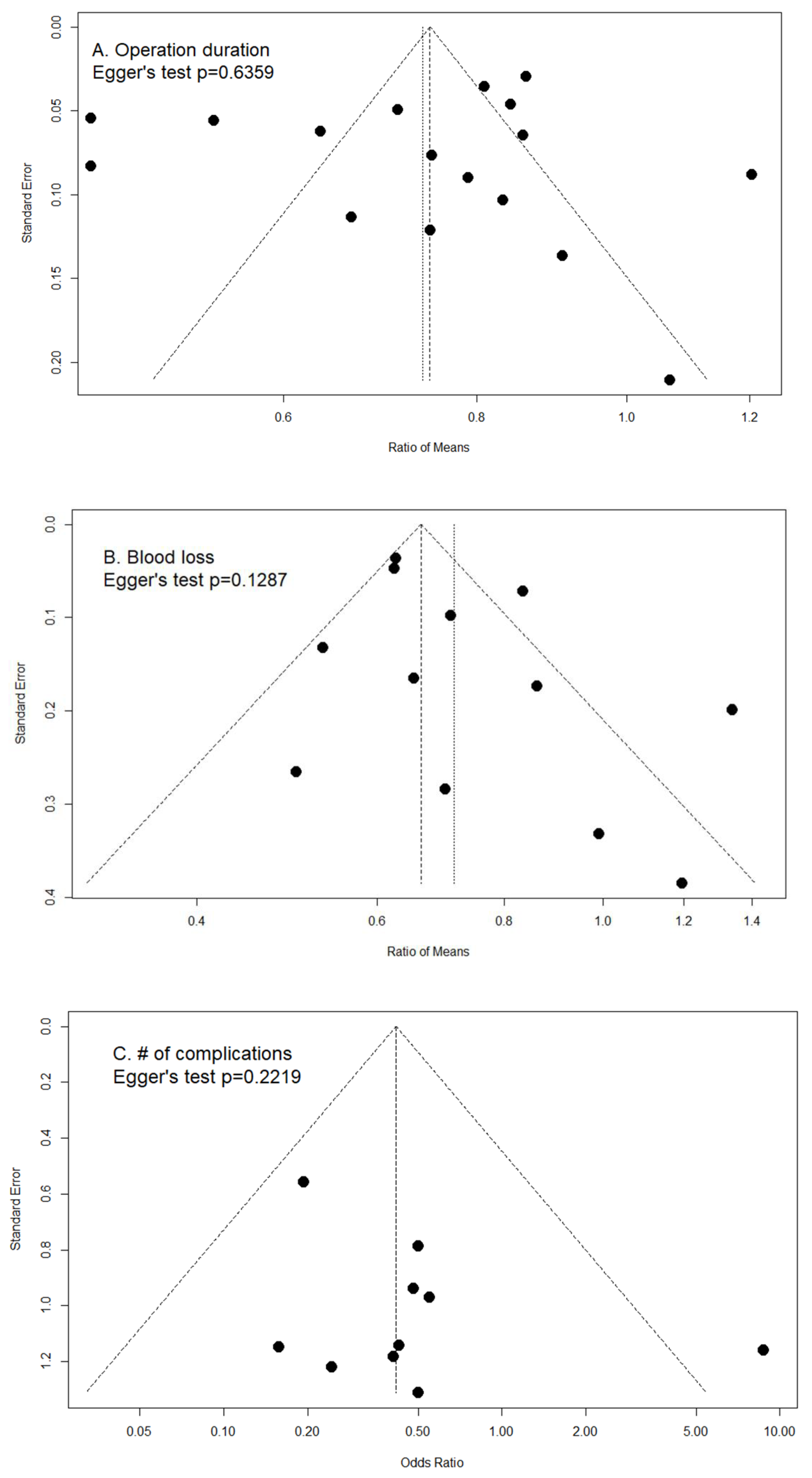
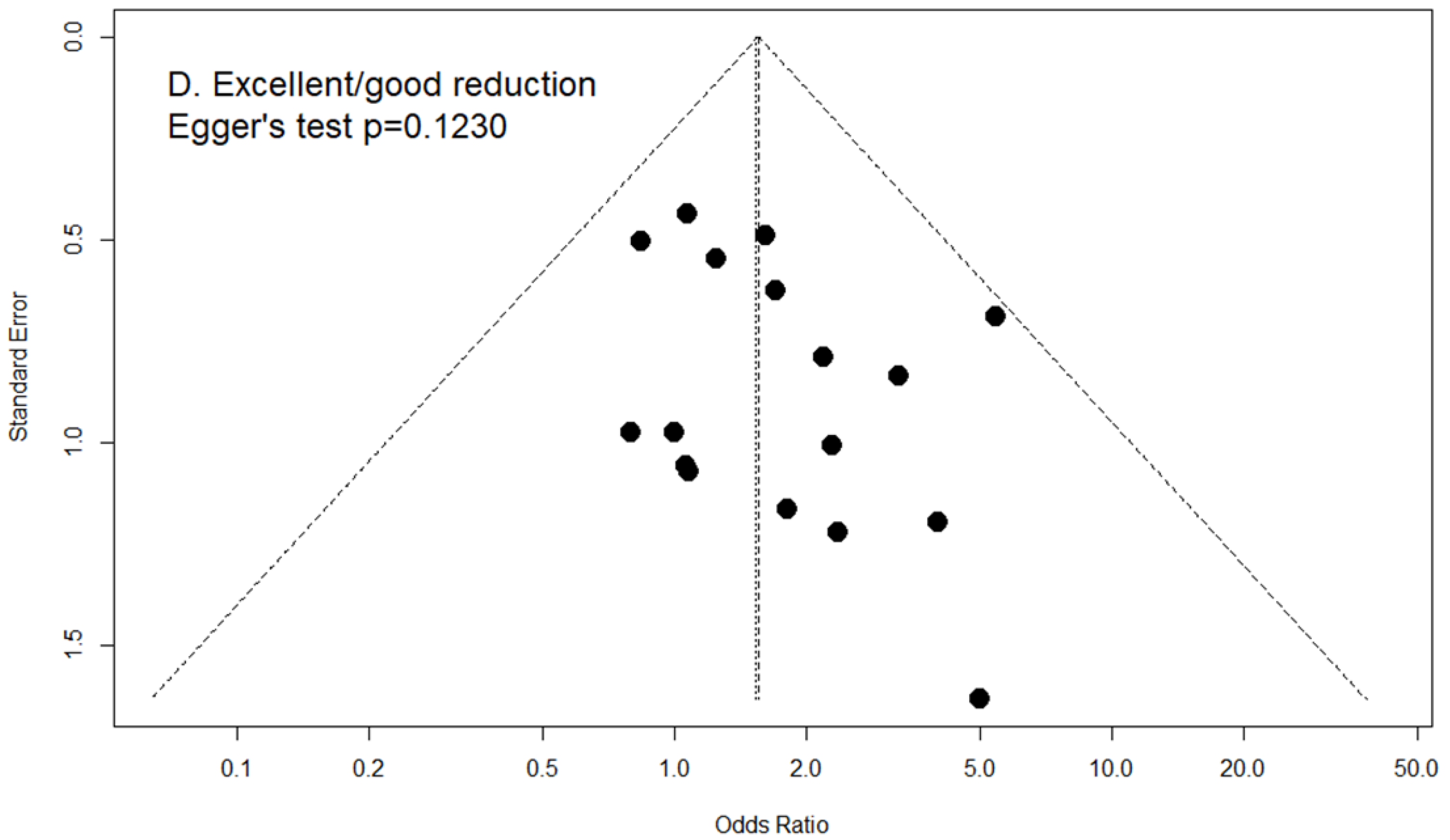
2.8. Publication Bias
3. Results
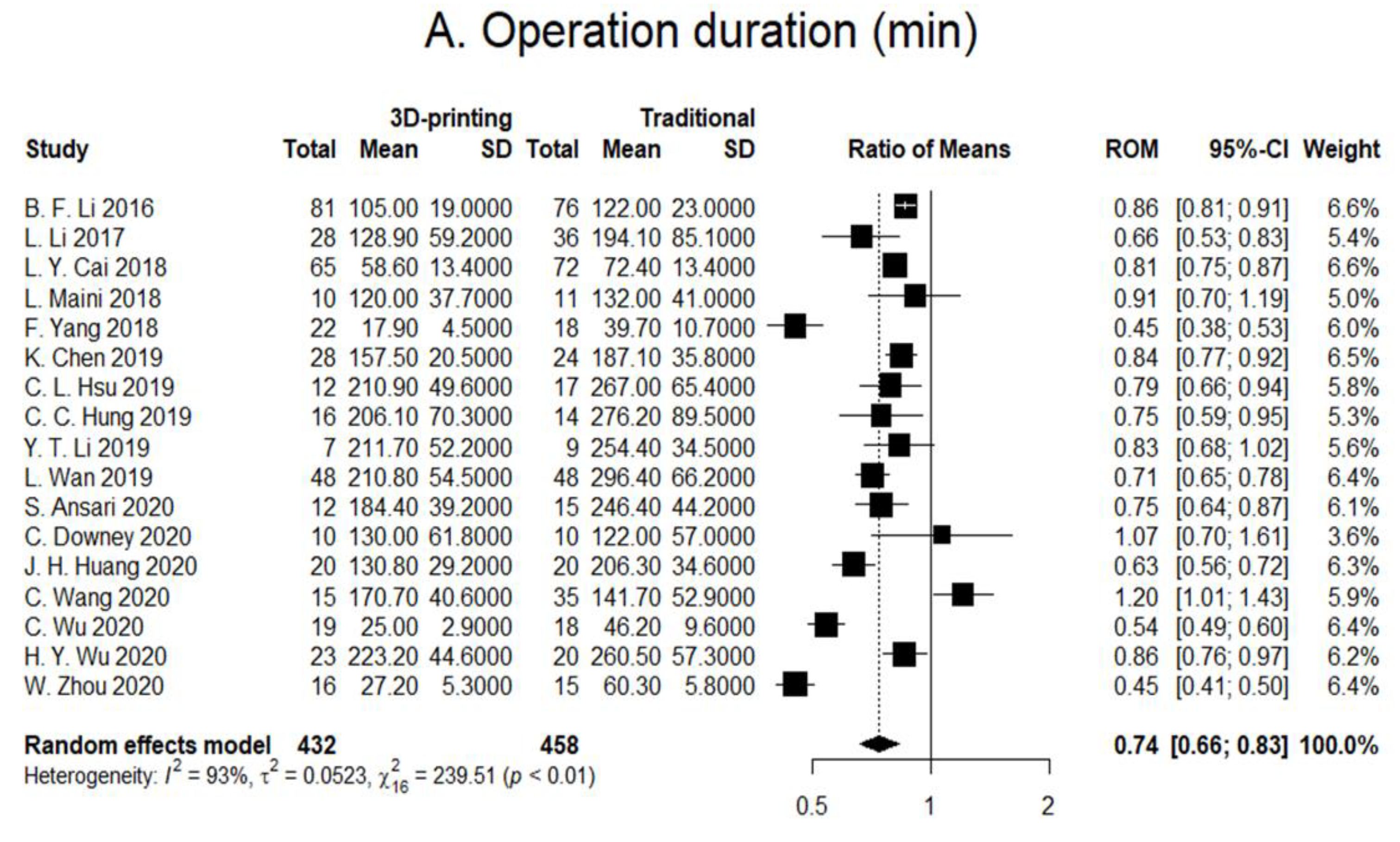
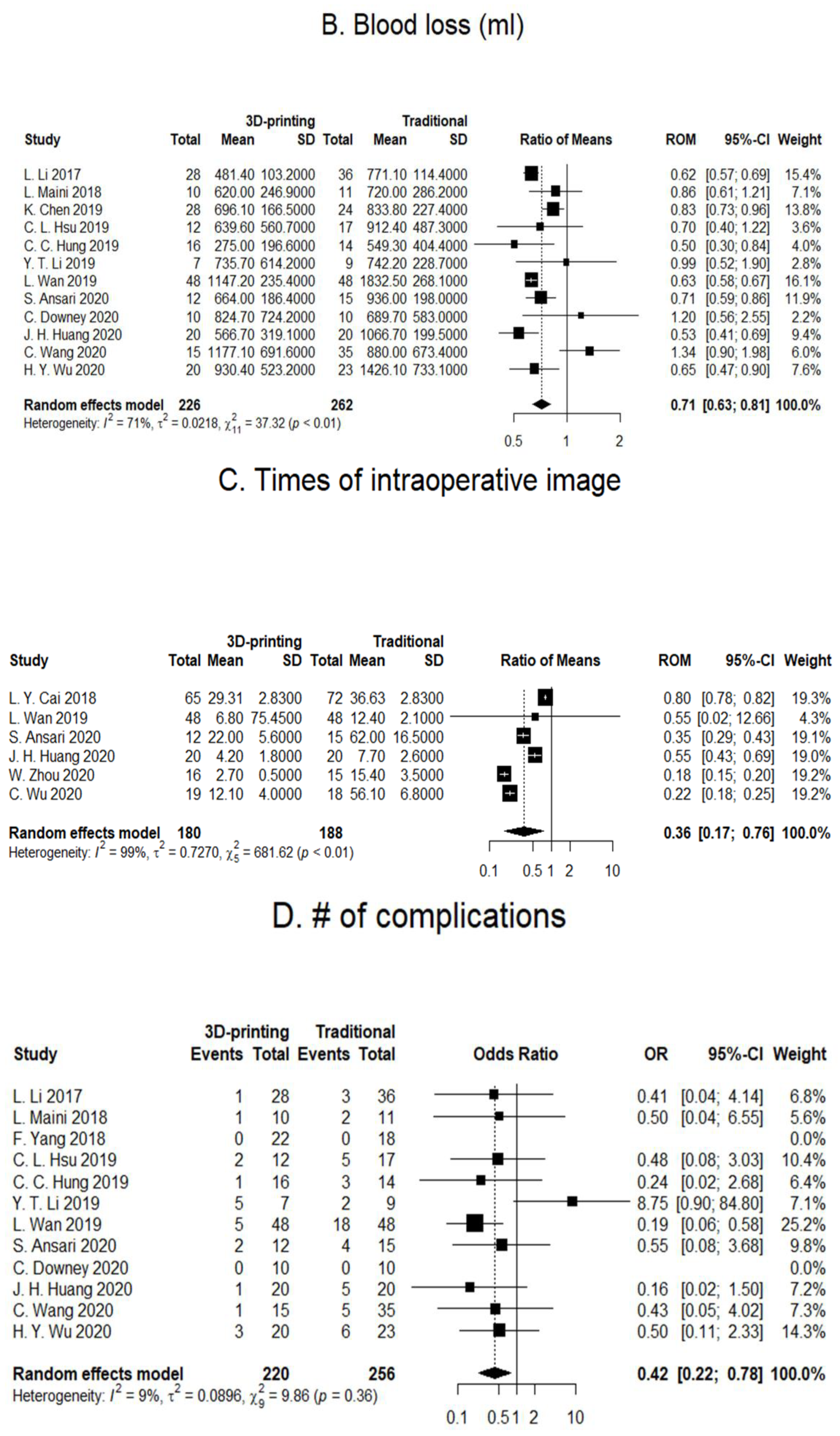
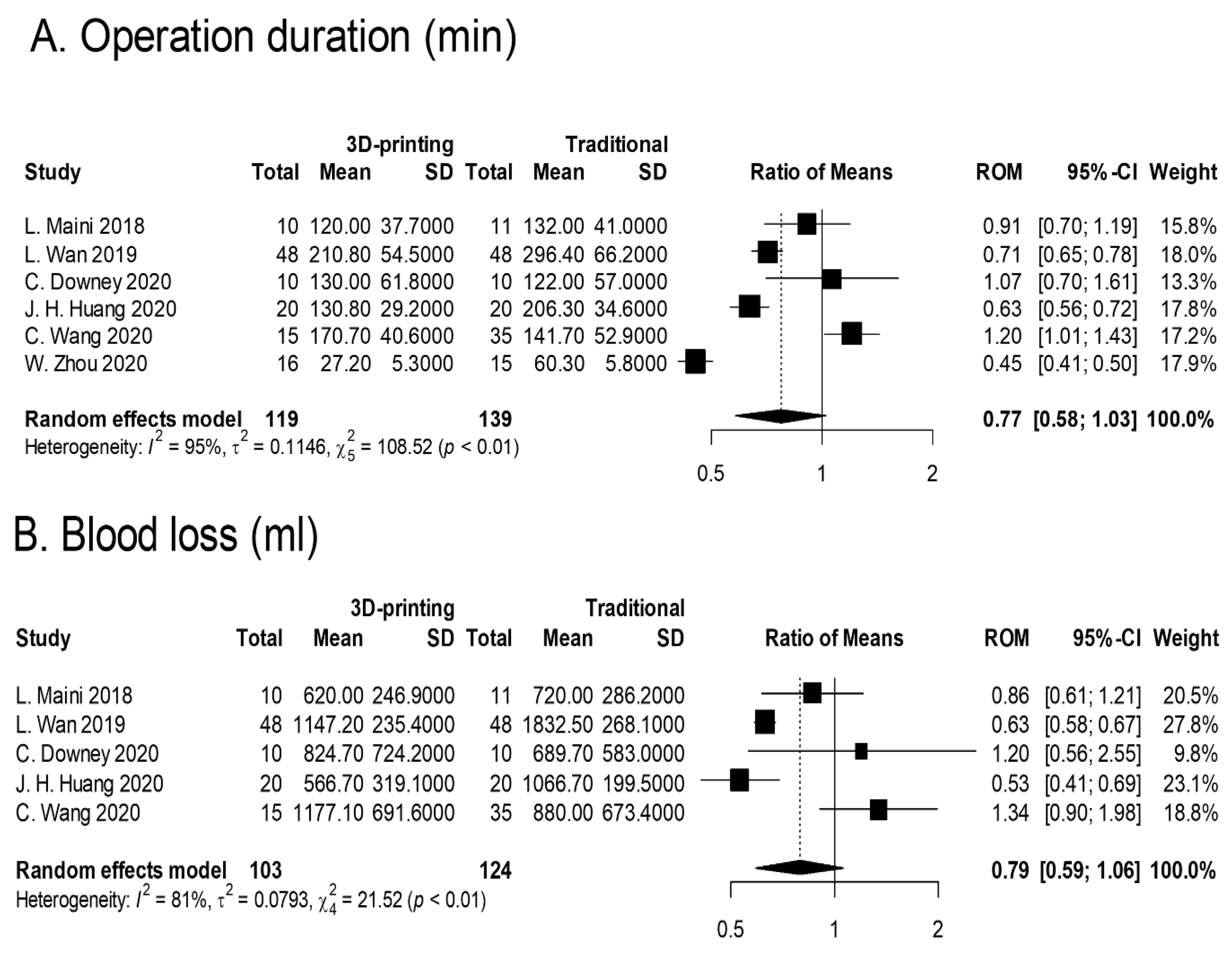
3.1. Operation Duration
3.2. Blood Loss
3.3. Intraoperative X-ray Exposure
3.4. Number of Postoperative Complications
3.5. Excellent/Good Reduction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, S.; Barik, S.; Singh, S.K.; Sarkar, B.; Goyal, T.; Kalia, R.B. Role of 3D printing in the management of complex acetabular fractures: A comparative study. Eur. J. Trauma Emerg. Surg. 2021, 47, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, J.; Bi, L.; Yuan, Z.; Pei, G. Comparison of three-dimensional printing and conventional imaging in surgical treatment of Tile C pelvic fractures: A long-term follow-up study. Int. J. Clin. Exp. Med. 2017, 10, 12433–12439. [Google Scholar]
- Zhou, W.; Xia, T.; Liu, Y.; Cao, F.; Liu, M.; Liu, J.; Mi, B.; Hu, L.; Xiong, Y.; Liu, G. Comparative study of sacroiliac screw placement guided by 3D-printed template technology and X-ray fluoroscopy. Arch. Orthop. Trauma Surg. 2020, 140, 11–17. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, H.; Gao, C. A Systematic Review and Meta-Analysis of 3D Printing Technology for the Treatment of Acetabular Fractures. BioMed. Res. Int. 2021, 2021, 5018791. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Liao, H.; Tan, X.Y.; Xing, W.R.; Zhou, Q.; Zheng, Y.S.; Cao, H.Y.; Zeng, C.J. Surgical treatment for both-column acetabular fractures using pre-operative virtual simulation and three-dimensional printing techniques. Chin. Med. J. 2020, 133, 395–401. [Google Scholar] [CrossRef]
- Bagaria, V.; Deshpande, S.; Rasalkar, D.D.; Kuthe, A.; Paunipagar, B.K. Use of rapid prototyping and three-dimensional reconstruction modeling in the management of complex fractures. Eur. J. Radiol. 2011, 80, 814–820. [Google Scholar] [CrossRef]
- Chen, K.; Yang, F.; Yao, S.; Xiong, Z.; Sun, T.; Zhu, F.; Telemacque, D.; Drepaul, D.; Ren, Z.; Guo, X. Application of computer-assisted virtual surgical procedures and three-dimensional printing of patient-specific pre-contoured plates in bicolumnar acetabular fracture fixation. Orthop. Traumatol. Surg. Res. 2019, 105, 877–884. [Google Scholar] [CrossRef]
- Lal, H.; Patralekh, M.K. 3D printing and its applications in orthopaedic trauma: A technological marvel. J. Clin. Orthop. Trauma 2018, 9, 260–268. [Google Scholar] [CrossRef]
- Chen, H.T.; Wang, Y.C.; Hsieh, C.C.; Su, L.T.; Wu, S.C.; Lo, Y.S.; Chang, C.C.; Tsai, C.H. Trends and predictors of mortality in unstable pelvic ring fracture: A 10-year experience with a multidisciplinary institutional protocol. World J. Emerg. Surg. 2019, 14, 61. [Google Scholar] [CrossRef]
- Downey, C.; McCarrick, C.; Fenelon, C.; Murphy, E.P.; O’Daly, B.J.; Leonard, M. A novel approach using 3-D printing in the Irish National Centre for pelvic and acetabular surgery. Ir. J. Med. Sci. 2020, 189, 219–228. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chou, Y.C.; Li, Y.T.; Chen, J.E.; Hung, C.C.; Wu, C.C.; Shen, H.C.; Yeh, T.-T. Pre-operative virtual simulation and three-dimensional printing techniques for the surgical management of acetabular fractures. Int. Orthop. 2019, 43, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Shen, P.H.; Wu, J.L.; Cheng, Y.W.; Chen, W.L.; Lee, S.H.; Yeh, T.-T. Association between 3D Printing-Assisted Pelvic or Acetabular Fracture Surgery and the Length of Hospital Stay in Nongeriatric Male Adults. J. Pers. Med. 2022, 12, 573. [Google Scholar] [CrossRef] [PubMed]
- Ijpma, F.F.A.; Meesters, A.M.L.; Merema, B.B.J.; ten Duis, K.; de Vries, J.P.P.M.; Banierink, H.; Wendt, K.W.; Kraeima, J.; Witjes, M.J.H. Feasibility of Imaging-Based 3-Dimensional Models to Design Patient-Specific Osteosynthesis Plates and Drilling Guides. JAMA Netw. Open 2021, 4, e2037519. [Google Scholar] [CrossRef] [PubMed]
- Maini, L.; Verma, T.; Sharma, A.; Sharma, A.; Mishra, A.; Jha, S. Evaluation of accuracy of virtual surgical planning for patient-specific pre-contoured plate in acetabular fracture fixation. Arch. Orthop. Trauma Surg. 2018, 138, 495–504. [Google Scholar] [CrossRef]
- Hung, C.C.; Li, Y.T.; Chou, Y.C.; Chen, J.E.; Wu, C.C.; Shen, H.C.; Yeh, T.-T. Conventional plate fixation method versus pre-operative virtual simulation and three-dimensional printing-assisted contoured plate fixation method in the treatment of anterior pelvic ring fracture. Int. Orthop. 2019, 43, 425–431. [Google Scholar] [CrossRef]
- Jinsihan, N.; Jin, G. Recent developments of 3D-printing technique assisted surgery in the management of complex fractures. Int. J. Clin. Exp. Med. 2018, 11, 11578–11583. [Google Scholar]
- Kim, C.H.; Kim, J.W. Plate versus sacroiliac screw fixation for treating posterior pelvic ring fracture: A Systematic review and meta-analysis. Injury 2020, 51, 2259–2266. [Google Scholar] [CrossRef]
- Li, B.; Chen, B.; Zhang, Y.; Wang, X.; Wang, F.; Xia, H.; Yin, Q. Comparative use of the computer-aided angiography and rapid prototyping technology versus conventional imaging in the management of the Tile C pelvic fractures. Int. Orthop. 2016, 40, 161–166. [Google Scholar] [CrossRef]
- Li, Y.T.; Hung, C.C.; Chou, Y.C.; Chen, J.E.; Wu, C.C.; Shen, H.C.; Yeh, T.T. Surgical treatment for posterior dislocation of hip combined with acetabular fractures using preoperative virtual simulation and three-dimensional printing model-assisted precontoured plate fixation techniques. BioMed Res. Int. 2019, 2019, 3971571. [Google Scholar] [CrossRef]
- Wu, H.Y.; Shao, Q.P.; Song, C.J.; Shang, R.R.; Liu, X.M.; Cai, X. Hua. Personalized Three-Dimensional Printed Anterior Titanium Plate to Treat Double-Column Acetabular Fractures: A Retrospective Case-Control Study. Orthop. Surg. 2020, 12, 1212–1222. [Google Scholar] [CrossRef]
- Yang, F.; Yao, S.; Chen, K.F.; Zhu, F.Z.; Xiong, Z.K.; Ji, Y.H.; Sun, T.F.; Guo, X.D. A novel patient-specific three-dimensional-printed external template to guide iliosacral screw insertion: A retrospective study. BMC Musculoskelet. Disord. 2018, 19, 397. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, Y.; Chen, C.; Lou, Y.; Guo, X.; Wang, J. 3D printing-based minimally invasive cannulated screw treatment of unstable pelvic fracture. J. Orthop. Surg. Res. 2018, 13, 71. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, X.; Zhang, S.; Li, K.; Cao, P.; Li, J.; Wu, G. Clinical feasibility and application value of computer virtual reduction combined with 3D printing technique in complex acetabular fractures. Exp. Ther. Med. 2019, 17, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Wang, L.; Wang, D.; Gu, C.; Lin, X.; Liu, H.; Chen, J.; Wen, X.; Liu, Y.; et al. Three-dimensional printing of patient-specific plates for the treatment of acetabular fractures involving quadrilateral plate disruption. BMC Musculoskelet. Disord. 2020, 21, 451. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Deng, J.Y.; Li, T.; Tan, L.; Yuan, D.C. Combined 3D Printed Template to Guide Iliosacral Screw Insertion for Sacral Fracture and Dislocation: A Retrospective Analysis. Orthop. Surg. 2020, 12, 241–247. [Google Scholar] [CrossRef]
- Liu, Z.J.; Jia, J.; Zhang, Y.G.; Tian, W.; Jin, X.; Hu, Y.C. Internal fixation of complicated acetabular fractures directed by preoperative surgery with 3D printing models. Orthop. Surg. 2017, 9, 257–260. [Google Scholar] [CrossRef]
- Maini, L.; Mishra, A.; Agarwal, G.; Verma, T.; Sharma, A.; Tyagi, A. 3D printing in designing of anatomical posterior column plate. J. Clin. Orthop. Trauma 2018, 9, 236–240. [Google Scholar] [CrossRef]
- Maini, L.; Sharma, A.; Jha, S.; Sharma, A.; Tiwari, A. Three-dimensional printing and patient-specific pre-contoured plate: Future of acetabulum fracture fixation? Eur. J. Trauma Emerg. Surg. 2018, 44, 215–224. [Google Scholar] [CrossRef]
- Morgan, C.; Khatri, C.; Hanna, S.A.; Ashrafian, H.; Sarraf, K.M. Use of three-dimensional printing in preoperative planning in orthopaedic trauma surgery: A systematic review and meta-analysis. World J. Orthop. 2020, 11, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Upex, P.; Jouffroy, P.; Riouallon, G. Application of 3D printing for treating fractures of both columns of the acetabulum: Benefit of pre-contouring plates on the mirrored healthy pelvis. Orthop. Traumatol. Surg. Res. 2017, 103, 331–334. [Google Scholar] [CrossRef]
- Weidert, S.; Andress, S.; Linhart, C.; Suero, E.M.; Greiner, A.; Böcker, W.; Kammerlander, C.; Becker, C.A. 3D printing method for next-day acetabular fracture surgery using a surface filtering pipeline: Feasibility and 1-year clinical results. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 565–575. [Google Scholar] [CrossRef]
- Wu, J.; Luo, D.; Xie, K.; Wang, L.; Hao, Y. Design and Application of 3D Printing Based Personalised Pelvic Prostheses. J. Shanghai Jiaotong Univ. (Sci.) 2021, 26, 361–367. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, C.; Fang, Y. Mapping of both column acetabular fractures with three-dimensional computed tomography and implications on surgical management. BMC Musculoskelet. Disord. 2019, 20, 255. [Google Scholar] [CrossRef]
- Blum, A.; Gillet, R.; Rauch, A.; Urbaneja, A.; Biouichi, H.; Dodin, G.; Germain, E.; Lombard, C.; Jaquet, P.; Louis, M.; et al. 3D reconstructions, 4D imaging and postprocessing with CT in musculoskeletal disorders: Past, present and future. Diagn. Interv. Imaging 2020, 101, 693–705. [Google Scholar] [CrossRef]




| Study Characteristics | Design | Sample Sizes (Total/ Control/Treatment) | Population Characteristics | Fracture Mapping and 3D Printing Technology Used | Key Operative Outcomes | Duration of Follow-Ups |
| B.F. Li, 2016, China, [18] | Retrospective | 157/76/81 Tile C pelvic fracture only Preoperative: CT | Age: Control: 32.2 ± 9.4 Treatment: 34.0 ± 8.2 Gender: Control: 51/25 Treatment: 55/26 ISS: Control: 19.6 ± 11.2 Treatment: 16.7 ± 14.7 | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Fractured pelvis with soft tissues Preoperative: Pre-contouring of plates, approach, and entering position of screws. | Surgical related outcomes: Improved surgical duration (122 ± 23 vs. 105 ± 19), anesthesia duration (155 ± 26 vs. 135 ± 28), Hb (6.2 ± 7.4 vs. 31.0 ± 8.2), blood transfusion (9.9% vs. 19.7%), PONV (8% vs. 10%), postoperative pain (2.5 ± 2.3 vs. 2.8 ± 2.0), length of hospital stay (7.8 ± 2.0 vs. 10.2 ± 3.1). Functional outcome: more patients were discharged home rather than to rehabilitation | No long-term follow-ups |
| L. Li, 2017, China, [2] | Retrospective | 64/36/28 Tile C pelvic fracture only Preoperative: X-ray and 2D CT | Age: Control: 34.5 ± 8.4 Treatment: 32.4 ± 7.6 Gender: Control: 28/8 Treatment: 18/10 ISS: Control: 17.2 ± 12.1 Treatment: 18.4 ± 15.3 | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Fractured pelvis with no soft tissues. Preoperative: Pre-contouring of plates, selection of incision approach, and entering position of screws. | Surgical related outcomes: Improved surgical duration (128.9 ± 59.2 vs. 194.1 ± 85.1) blood loss (481.4 ± 103.2 vs. 771.29 ± 114.4), blood transfusion (345.1 ± 75.4 vs. 736.6 ± 125.9). Functional outcomes: Improved complications (1 vs. 3) Matta (excellent/good/poor: 20/6/2 vs. 8/16/12), Majeed (excellent/good/fair at 1 year: 20/6/2 vs. 18/7/11; 10 yr: 18/6/4 vs. 18/6/12) | Up to 10 years |
| F. Yang, 2018, China, [21] | Retrospective | 40/18/22 Traumatic incomplete or complete disruptions of the posterior pelvic ring (types 54B and 54C) 54B/54C Control: 4/14 Treatment: 7/15 Preoperative: CT | Age: Control: 50.1 ± 13.7 Treatment: 51.7 ± 15.2 Gender: Control: 10/8 Treatment: 11/11 ISS: Control: 17.2 ± 12.1 Treatment: 18.4 ± 15.3 | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Template guide Preoperative: Virtual simulation of screw placement with printing of screw template guide. | Surgical related outcomes: Improved surgical duration (17.9 ± 4.5 vs. 39.7 ± 10.7), amount of radioactive exposure (742.8 ± 230.6 vs. 1904.0 ± 844.5 cGy), rate of screw perforation (1 of 37 vs. 4 of 38). Functional outcomes: Reduction quality (excellent/good: 7/12 vs. 5/11). | No long-term follow-ups |
| ** L. Maini, 2018, India, [14] | RCT | 21/11/10 Control: 62A1 (1), 62A2 (1), 62A3 (1), 62B1 (2), 62B2 (5) 62C1 (1) Treatment: 62A1 (2), 62B1(4), 62B2 (3) 62C1 (1) Preoperative: CT | Mean age: 38.7 Gender: 18/21 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Fractured pelvis with no soft tissues. Preoperative: Pre-contouring of plates. | Surgical related outcomes: Surgical duration (120 ± 37.7 vs. 132 ± 41) blood loss (620 ± 246.9 vs. 720 ± 286.2), additional instrumentation tines (9 vs. 0 min) Functional outcomes: Improved complications (1 vs. 2), CT residual displacement (4.75 ± 3.13 vs. 7.60 ± 4.92 mm), CT reduction (anatomic: 1 vs. 4). | No long-term follow-ups |
| L.Y. Cai, 2018, China, [22] | Retrospective | 137/72/65 Tile B and C pelvic fracture Preoperative: CT | Age: Control: 32.63 ± 4.72 Treatment: 33.08 ± 4.91 Gender: Control: 45/27 Treatment: 37/28 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Mirroring of contralateral healthy pelvis. Preoperative: Pre-contouring of plates and K-wires. | Surgical related outcomes: Surgical duration (58.63 ± 13.38 vs. 72.38 ± 13.4), timing of radioactive exposure (29.31 ± 2.83 vs. 36.63 ± 2.83). Functional outcome: Fracture healing time (13.8 ± 1.96 vs. 14.5 ± 1.56 weeks), Majeed (excellent: 80.6% vs. 78.5%) and Matta (excellent: 84% vs. 81.5%). | No long-term follow-ups |
| C.C. Hung, 2018, Taiwan, [15] | Retrospective | 30/14/16 Control: Tile A (1), Tile B (8), Tile C (5) Treatment: Tile A (1), Tile B (6), Tile C (9) Preoperative: CT | Age: Control: 35.64 ± 17.37 Treatment: 35.44 ± 13.52 Gender: Control: 8/6 Treatment: 10/6 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Mirroring of contralateral healthy pelvis Preoperative: Pre-contouring of plates and selection of screw length | Surgical related outcomes: Improved surgical duration (206.13 ± 70.32 vs. 276.21 ± 89.53), blood loss (45.6 ± 15.26 vs. 549.29 ± 404.43), instrumentation time (45.63 ± 15.26 vs. 102.86 ± 25.85). Functional outcome: Complications (1 vs. 3), CT reduction (good: 13 vs. 8). | 2 years |
| C.L. Hsu, 2018, Taiwan, [11] | Retrospective | 29/17/12 Control: Posterior wall (5), both columns (4), transverse with posterior wall (2), posterior column with posterior wall (1), T-shaped with posterior wall (4), both columns with posterior wall (1) Treatment: Posterior wall (3), posterior column (1), transverse (1), both columns (2), transverse with posterior wall (2), posterior column with posterior wall (1), T-shaped with posterior wall (1), both columns with posterior wall (1) Preoperative: CT | Age: Control: 38.24 ± 16.39 Treatment: 36.75 ± 16.39 Gender: Control: 14/3 Treatment: 11/1 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Mirroring of contralateral healthy pelvis Preoperative: Surgical plan (including plate number, position, length, curvature, and screw position) and simulation of surgery with printed model. | Surgical related outcomes: Improved surgical duration (posterior column: 222.75 ± 48.12 vs. 259.76 ± 46.50), blood loss (anterior column: 433.33 ± 317.28 vs. 958.33 ± 427.10), instrumentation time (anterior column: 43.40 ± 10.92 vs. 99.00 ± 15.44; posterior column: 35.75 ± 9.21 vs. 67.35 ± 10.8). Surgical related outcomes: Surgical duration (anterior column: 199 ± 50.29 vs. 274.17 ± 80.95), blood loss (posterior column: 845.83 ± 681.06 vs. 866.47 ± 550.33) Functional outcome: Cmplications (2 vs. 5), X-ray reduction (good: 14 vs. 11). | Mean: 14.4 months (3–43 months) |
| K. Chen, 2019, China, [7] | Retrospective | 52/24/28 T-shaped (10), anterior column with posterior hemi-transverse (16), double column (24). Preoperative: CT | Age: Control: 42.38 ± 12.28 Treatment: 46.11 ± 13.63 Gender: Control: 14/10 Treatment: 18/10 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Mirroring of contralateral healthy pelvis. Preoperative: Pre-contouring of plates. | Surgical related outcomes: Improved surgical duration (157.5 ± 20.48 vs. 187.08 ± 35.81), blood loss (696.07 ± 166.54 vs. 833.75 ± 227.44). Functional outcome: Merle d’Aubigné (16.25 ± 1.65 vs. 15.83 ± 1.88) and Matta (excelent/good: 19/24 vs. 25/28). | Not mentioned |
| ** L. Wan, 2019, China, [23] | Non-randomized controlled trials | 96/48/48 Control: T-shaped (8), posterior column with posterior wall (11), both columns (9), transverse with posterior wall (12), anterior with transverse (6), marginal(1), compression fracture(1). Treatment: T-shaped (7), posterior column with posterior wall (12), both columns (8), transverse with posterior wall (11), anterior with transverse (7), marginal (2), compression fracture (2) Preoperative: CT | Age: Control: 41.88 ± 4.97 Treatment: 43.44 ± 4.53 Gwender: Control: 32/16 Treatment: 34/14 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Both reduced and fractured pelvis models Preoperative: Simulation, selection of incision approach, sequence of reduction, placement of reduction clamp, rotation direction, placement of plate and screw, angle, length and pre-contouring of plates. | Surgical related outcomes: Improved surgical duration (150.24 ± 75.45 vs. 296. ± 76.83), blood loss (1147.2 ± 235.4 vs. 1832.5 ± 268), timing of radioactive exposure (6.8 ± 75.45 vs. 12.4 ± 2.1). Functional outcome: Improved complications (5 vs. 18). Functional outcomes: CT reduction (excellent: 81.25% vs. 77.08%), hip joint function (excellent: 87.5% vs. 83.3%) | Up to 6 months |
| Y.T. Li, 2019, Taiwan [19] | Retrospective | 16/9/7 Control: T-shaped with posterior wall (2), posterior column with posterior wall (1), posterior wall (6) Treatment: T-shaped with posterior wall (1), posterior column with posterior wall (1), posterior wall (3), transverse with posterior wall (2) Preoperative: CT | Age: Control: 37.00 ± 17.09 Treatment: 32.14 ± 14.63 Gender: Control: 6/3 Treatment: 7/0 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Mirroring of contralateral healthy pelvis onto fractured model simulation. Preoperative: Surgical plan (including the type of plate and plate number, curvature, position, and screw length) with pre-contouring of plates. | Surgical related outcomes: Surgical duration (211.71 ± 52.23 vs. 254.44 ± 34.46), blood loss (735.71 ± 614.22 vs. 742.22 ± 228.68). Surgical related outcomes: Improved instrumentation timing (38.43 ± 10.81 vs. 71.78 ± 9.69). Functional outcome: Complications (2 vs. 5), CT residual displacement (<2 mm: 7 vs. 7). | No long-term follow-ups |
| ** C. Downey, 2020, Ireland, [10] | Non-randomized controlled trials | 20/10/10 Anterior column posterior hemi-transverse (8), both columns (8), T-shaped (2), lateral compression complex (2). Preoperative: CT | Age: Control: 51.8 ± 14.9 Treatment: 51.9 ± 18.9 Gender: Control: 9/1 Treatment: 9/1 ISS: Control: 18.8 ± 3.8 Treatment: 20.6 ± 8 | 3D image processing software: Meshmixer 3D printed model: Fractured pelvis Preoperative: Pre-operative planning | Surgical related outcomes: Surgical duration (122 vs. 130), blood loss (689.7 vs. 824.7), radioactive exposure (727.1 vs. 1078.1 cGy), Functional outcome: similar EQ-5D-5L scores, reduction (anatomical: 10% vs. 10%) Others: Surgeon questionnaire | 12 months |
| ** C. Wang, 2020, China, [24] | Non-randomized controlled trials | 50/35/15 Control: both columns (22), anterior column posterior hemi-transverse (10), T-shaped (3) Treatment: both columns (11), anterior column posterior hemi-transverse (3), T-shaped (1) Preoperative: CT | Age: Control: 45.1 ± 12.6 Treatment: 46.6 ± 12.3 Gender: Control: 22/13 Treatment: 10/5 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Mirroring of the contralateral healthy acetabulum Preoperative: 3D printed plates and screws with simulation | Surgical related outcomes: Improved surgical duration (141.7 ± 52.9 vs. 170.7 ± 40.6), blood loss (880.0 ± 673.4 vs. 1177.1 ± 691.6). Functional outcome: Improved residual displacement (1.51 ± 0.97 vs. 2.38 ± 1.10 mm) Functional outcome: Complications (1 vs. 5), Matta (anatomical/satisfactory: 10/4 vs. 18/13). | Not mentioned |
| C. Wu, 2019, China, [25] | Retrospective | 37/18/19 Control: Tile B (15), Tile C (3) Treatment: Tile B (15), Tile (4) Preoperative: CT | Age: Control: 42.4 ± 5.7 Treatment: 43.1 ± 12.7 Gender: Control: 12/6 Treatment: 12/7 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Fractured pelvis model with template guide | Surgical related outcomes: Improved surgical duration per screw (25 ± 2.9 vs. 46.2 ± 9.6), radiation exposure (12.1 ± 4 vs. 56.1 ± 6.8). Functional outcome: Improved grading (I/II: 40%/2% vs. 26%/3%). Functional outcome: Reduction (excellent/good: 6/11 vs. 5/11). | 6 months |
| H.Y. Wu, 2020, China, [20] | Retrospective | 43/20/23 Double column acetabular fracture Preoperative: CT | Age: Control: 50.1 ± 8.2 Treatment: 51.0 ± 8.6 Gender: Control: 15/5 Treatment: 16/7 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Mirroring of the contralateral healthy pelvis with template guide | Surgical related outcomes: Improved surgical duration per screw (260.5 ± 57.3 vs. 223.2 ± 44.6), blood loss (1426.1 ± 733.1 vs. 930.4 ± 523.2). Functional outcome: Complications (3 vs. 6), reduction (anatomical: 13 vs. 12), Merle d’Aubigné (excellent: 11 vs. 11), duration of hospital stay (24.6 ± 4.9 vs. 26.4 ± 7.2). | Not mentioned |
| ** J.H. Huang, 2020, China, [5] | RCT | 40/20/20 Double column acetabular fracture Preoperative: CT | Age: Control: 37.4 ± 12.7 Treatment: 43.4 ± 11.6 Gender: Control: 14/6 Treatment: 12/8 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Reduced pelvis model Preoperative: Pre-contouring of plate and screw measurement. | Surgical related outcomes: Improved surgical duration (130.8 ± 29.2 vs. 206.3 ± 34.6), blood loss (500 vs. 1050), instrumentation time (32.1 ± 9.5 vs. 57.9 ± 15.1), radiation exposure (4.2 ± 1.8 vs. 7.7 ± 2.6 secs). Functional outcome: Complications (1 vs. 5), postoperative X-ray reduction (good: 13 vs. 8). Functional outcome: Improved bone union (14.48 ± 1.52 vs. 15.85 ± 1.56 weeks). | 1 to 5 months |
| S. Ansari, 2020, India, [1] | Retrospective | 27/15/12 Control: posterior column with posterior wall (6), transverse with posterior wall (4), double columns (3), anterior column posterior hemi-transverse (1), T-shaped (1) Treatment: posterior column with posterior wall (5), transverse with posterior wall (3), double columns (2), anterior column posterior hemi-transverse (1), T-shaped (1) Preoperative: CT | Age: Control: 39.1 ± 12.4 Treatment: 41.3 ± 13.7 Gender: Control: 12/3 Treatment: 11/1 ISS: Not mentioned | 3D image processing software: Syngo.via VB40 software (Siemens, Munich, Germany) 3D printed model: Mirroring of the contralateral healthy pelvis Preoperative: Pre-contouring of plate and screw measurement and surgical approach. | Surgical related outcomes: Improved surgical duration (184.4 ± 39.2 vs. 246.4 ± 44.2), blood loss (664 ± 186.4 vs. 936 ± 198.4), radiation exposure (22 ± 5.6 vs. 62 ± 16.5 min). Functional outcome: Complications (2 vs. 4), reduction (<2 mm: 11 vs. 11), Harris (79.7 ± 13.7 vs. 83.4 ± 12.3). | Not mentioned |
| ** W. Zhou, 2020, China, [3] | Non-randomized controlled trials | 31/15/16 Control: Tile C1 (13), Tile C2 (2) Treatment: Tile C1 (13), Tile C2 (2) Preoperative: CT | Age: Control: 47.1 ± 0.5 Treatment: 47.2 ± 0.8 Gender: Control: 11/4 Treatment: 11/5 ISS: Not mentioned | 3D image processing software: MIMICS (Materialise Interactive Medical Image Control System Software, Materialise, Belgium) 3D printed model: Reduced pelvis with template guide | Surgical related outcomes: Improved surgical duration per screw (27.2 ± 5.3 vs. 60.3 ± 5.8), radiation exposure (2.7 ± 0.5 vs. 15.4 ± 3.5). Functional outcome: Matta (excellent: 13 vs. 14), Majeed (excellent: 12 vs. 14). | 6 to 20 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.K.-X.; Lin, T.-L.; Hsu, C.-J.; Fong, Y.-C.; Chen, H.-T.; Tsai, C.-H. Three-Dimensional Printing and Fracture Mapping in Pelvic and Acetabular Fractures: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5258. https://doi.org/10.3390/jcm11185258
Lee AK-X, Lin T-L, Hsu C-J, Fong Y-C, Chen H-T, Tsai C-H. Three-Dimensional Printing and Fracture Mapping in Pelvic and Acetabular Fractures: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(18):5258. https://doi.org/10.3390/jcm11185258
Chicago/Turabian StyleLee, Alvin Kai-Xing, Tsung-Li Lin, Chin-Jung Hsu, Yi-Chin Fong, Hsien-Te Chen, and Chun-Hao Tsai. 2022. "Three-Dimensional Printing and Fracture Mapping in Pelvic and Acetabular Fractures: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 18: 5258. https://doi.org/10.3390/jcm11185258






