Metabolism and Intracranial Epileptogenicity in Temporal Lobe Long-Term Epilepsy-Associated Tumor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Neuroimaging Acquisition
2.3. Metabolic Pattern Analysis
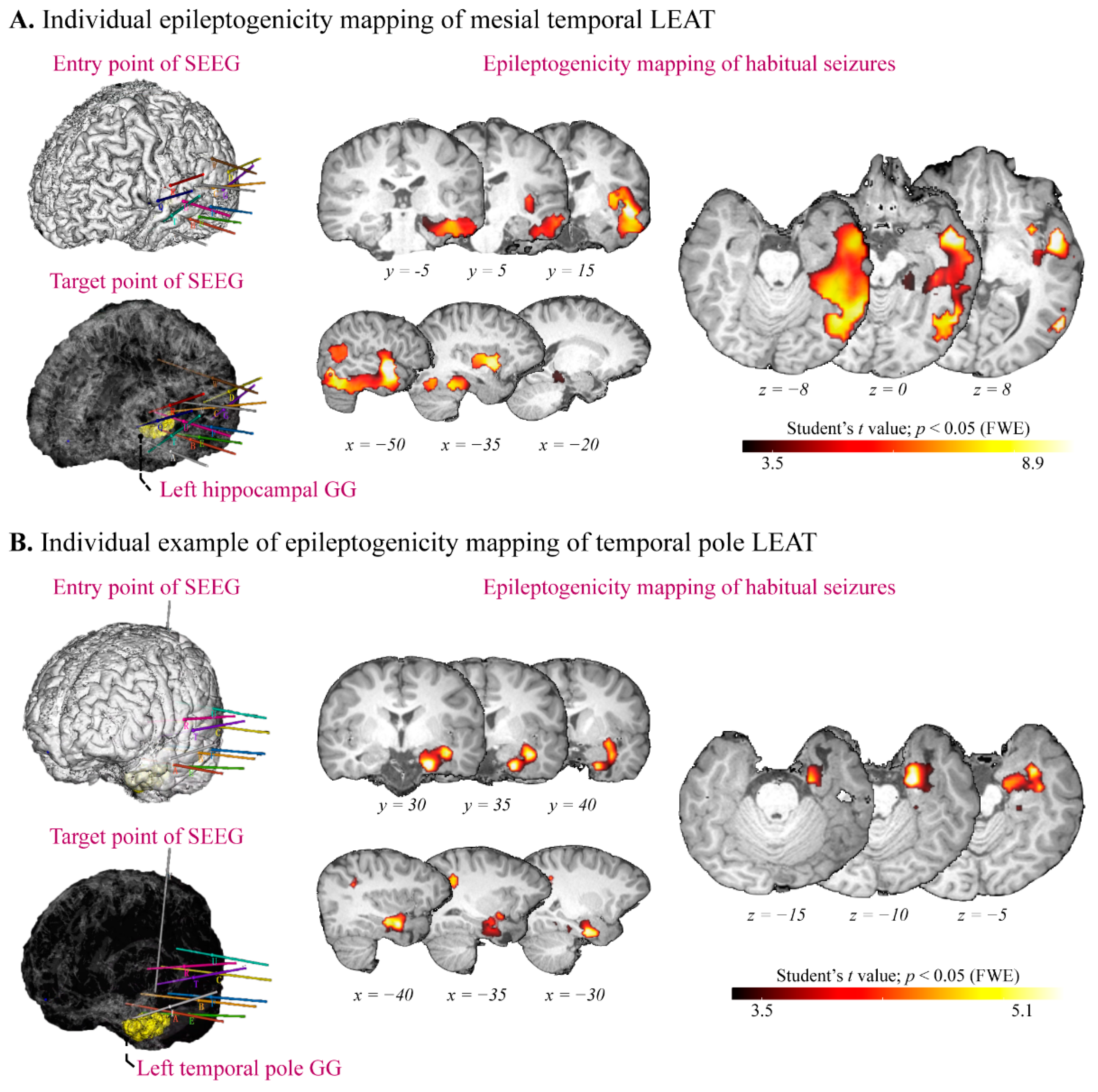
2.4. SEEG Recording
2.5. Epileptogenicity Mapping
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Mapping Metabolic Alteration to Controls
3.3. Subgroup Analysis of Metabolic Alteration
3.4. Epileptogenicity Mapping of Temporal Lobe LEAT
4. Discussion
4.1. Highlights
4.2. Metabolic Network of Temporal Lobe LEAT
4.3. SEEG Implantation and Epileptogenicity in Patients with Temporal Lobe LEAT
4.4. Surgical Indication for Tumor-Associated Temporal Lobe Epilepsy
4.5. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blumcke, I.; Spreafico, R.; Haaker, G.; Coras, R.; Kobow, K.; Bien, C.; Pfäfflin, M.; Elger, C.; Widman, G.; Schramm, J.; et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017, 377, 1648–1656. [Google Scholar] [CrossRef]
- Slegers, R.; Blumcke, I. Low-grade developmental and epilepsy associated brain tumors: A critical update 2020. Acta Neuropathol. Commun. 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, V.; Deleo, F.; Gozzo, F.; Sartori, I.; Mai, R.; Cossu, M.; Tassi, L. Early and late epilepsy surgery in focal epilepsies associated with long-term epilepsy-associated tumors. J. Neurosurg. 2017, 127, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- De Palma, L.; Pepi, C.; De Benedictis, A.; Pietrafusa, N.; Mastronuzzi, A.; Cacchione, A.; Carfì-Pavia, G.; Rossi-Espagnet, C.; Diomedi-Camassei, F.; Rossi, S.; et al. Early Onset Epilepsy Caused by Low-Grade Epilepsy-Associated Tumors and Focal Meningeal Involvement. Brain Sci. 2020, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.; Berger, M.; Barbaro, N.; Chang, E. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia 2012, 53, 51–57. [Google Scholar] [CrossRef]
- Schaller, K.; Cabrilo, I. Anterior temporal lobectomy. Acta Neurochir. 2016, 158, 161–166. [Google Scholar] [CrossRef]
- Boling, W. Surgical Considerations of Intractable Mesial Temporal Lobe Epilepsy. Brain Sci. 2018, 8, 35. [Google Scholar] [CrossRef]
- Alonso-Vanegas, M.; Freire Carlier, I.; San-Juan, D.; Martínez, A.; Trenado, C. Parahippocampectomy as a New Surgical Approach to Mesial Temporal Lobe Epilepsy Caused By Hippocampal Sclerosis: A Pilot Randomized Comparative Clinical Trial. World Neurosurg. 2018, 110, e1063–e1071. [Google Scholar] [CrossRef]
- Wagner, K.; Gau, K.; Metternich, B.; Geiger, M.J.; Wendling, A.S.; Kadish, N.E.; Reuner, G.; Mayer, H.; Mader, I.; Beck, J.; et al. Effects of hippocampus-sparing resections in the temporal lobe: Hippocampal atrophy is associated with a decline in memory performance. Epilepsia 2020, 61, 725–734. [Google Scholar] [CrossRef]
- Herlin, B.; Adam, C.; Habert, M.; Mathon, B.; Clemenceau, S.; Navarro, V.; Dupont, S. Temporal pole epilepsy surgery-Sparing the hippocampus. Epilepsia 2020, 61, e146–e152. [Google Scholar] [CrossRef]
- Morita-Sherman, M.; Louis, S.; Vegh, D.; Busch, R.; Ferguson, L.; Bingaman, J.; Bulacio, J.; Najm, I.; Jones, S.; Zajichek, A.; et al. Outcomes of resections that spare vs remove an MRI-normal hippocampus. Epilepsia 2020, 61, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- Giulioni, M.; Marucci, G.; Martinoni, M.; Marliani, A.; Toni, F.; Bartiromo, F.; Volpi, L.; Riguzzi, P.; Bisulli, F.; Naldi, I.; et al. Epilepsy associated tumors: Review article. World J. Clin. Cases 2014, 2, 623–641. [Google Scholar] [CrossRef]
- Montaz-Rosset, M.; Scholly, J.; Voulleminot, P.; Severac, F.; Hirsch, E.; Valenti-Hirsch, M.; Namer, I. Comparison of Functional Deficit Zone Defined by FDG PET to the Epileptogenic Zones Described in Stereo-Electroencephalograph in Drug-Resistant Epileptic Patients Treated by Surgery. Clin. Nucl. Med. 2019, 44, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Taussig, D.; Petrescu, A.; Aghakhani, N.; Herbrecht, A.; Dorfmüller, G.; Ferrand-Sorbets, S.; Chipaux, M.; Fohlen, M.; Rodrigo, S.; Bouilleret, V. F-FDG-PET hypometabolic pattern reveals multifocal epileptic foci despite limited unique stereotyped seizures. Epilepsy Res. 2021, 172, 106589. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Thom, M.; Aronica, E.; Armstrong, D.; Vinters, H.; Palmini, A.; Jacques, T.; Avanzini, G.; Barkovich, A.; Battaglia, G.; et al. The clinicopathologic spectrum of focal cortical dysplasias: A consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011, 52, 158–174. [Google Scholar] [CrossRef]
- Bartolomei, F.; Chauvel, P.; Wendling, F. Epileptogenicity of brain structures in human temporal lobe epilepsy: A quantified study from intracerebral EEG. Brain A J. Neurol. 2008, 131, 1818–1830. [Google Scholar] [CrossRef]
- Mo, J.; Liu, Z.; Sun, K.; Ma, Y.; Hu, W.; Zhang, C.; Wang, Y.; Wang, X.; Liu, C.; Zhao, B.; et al. Automated detection of hippocampal sclerosis using clinically empirical and radiomics features. Epilepsia 2019, 60, 2519–2529. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, J.; Li, W.; Chen, C.; Zhou, N.; Hu, W.; Zhang, C.; Wang, Y.; Wang, X.; Liu, C.; et al. Clinical Value of Machine Learning in the Automated Detection of Focal Cortical Dysplasia Using Quantitative Multimodal Surface-Based Features. Front. Neurosci. 2018, 12, 1008. [Google Scholar] [CrossRef]
- Ashburner, J. SPM: A history. NeuroImage 2012, 62, 791–800. [Google Scholar] [CrossRef]
- David, O.; Blauwblomme, T.; Job, A.; Chabardès, S.; Hoffmann, D.; Minotti, L.; Kahane, P. Imaging the seizure onset zone with stereo-electroencephalography. Brain A J. Neurol. 2011, 134, 2898–2911. [Google Scholar] [CrossRef]
- Lamusuo, S.; Jutila, L.; Ylinen, A.; Kälviäinen, R.; Mervaala, E.; Haaparanta, M.; Jääskeläinen, S.; Partanen, K.; Vapalahti, M.; Rinne, J. [18F] FDG-PET reveals temporal hypometabolism in patients with temporal lobe epilepsy even when quantitative MRI and histopathological analysis show only mild hippocampal damage. Arch. Neurol. 2001, 58, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Chassoux, F.; Artiges, E.; Semah, F.; Desarnaud, S.; Laurent, A.; Landre, E.; Gervais, P.; Devaux, B.; Helal, O. Determinants of brain metabolism changes in mesial temporal lobe epilepsy. Epilepsia 2016, 57, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Jaisani, Z.; Miletich, R.; Ramanathan, M.; Weinstock, A. Clinical FDG-PET Findings in Patients with Temporal Lobe Epilepsy: Concordance with EEG and MRI. J. Neuroimag. Off. J. Am. Soc. Neuroimag. 2020, 30, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, B.; Hu, W.; Zhang, C.; Wang, X.; Wang, Y.; Liu, C.; Mo, J.; Sang, L.; Ma, Y.; et al. Effective connectivity among the hippocampus, amygdala, and temporal neocortex in epilepsy patients: A cortico-cortical evoked potential study. Epilepsy Behav. EB 2021, 115, 107661. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhao, X.; Liu, J.; Yao, X.; Hou, F.; Xu, Y.; Feng, Q. Functional connectivity changes of nucleus Accumbens Shell portion in left mesial temporal lobe epilepsy patients. Brain Imaging Behav. 2020, 14, 2659–2667. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Sumi, Y.; Sako, K.; Nihira, A.; Nakagawara, J. Putaminal hyperperfusion in dystonic posturing on subtracted SPECT: A case report. Ann. Nucl. Med. 2001, 15, 255–257. [Google Scholar] [CrossRef]
- Bernhardt, B.; Bernasconi, N.; Kim, H.; Bernasconi, A. Mapping thalamocortical network pathology in temporal lobe epilepsy. Neurology 2012, 78, 129–136. [Google Scholar] [CrossRef]
- Mo, J.; Wang, Y.; Zhang, J.; Cai, L.; Liu, Q.; Hu, W.; Sang, L.; Zhang, C.; Wang, X.; Shao, X.; et al. Metabolic phenotyping of hand automatisms in mesial temporal lobe epilepsy. EJNMMI Res. 2022, 12, 32. [Google Scholar] [CrossRef]
- Fan, L.; Wang, J.; Zhang, Y.; Han, W.; Yu, C.; Jiang, T. Connectivity-based parcellation of the human temporal pole using diffusion tensor imaging. Cereb. Cortex 2014, 24, 3365–3378. [Google Scholar] [CrossRef]
- Barba, C.; Rheims, S.; Minotti, L.; Guénot, M.; Hoffmann, D.; Chabardès, S.; Isnard, J.; Kahane, P.; Ryvlin, P. Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain A J. Neurol. 2016, 139, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Lagarde, S.; Boucekine, M.; McGonigal, A.; Carron, R.; Scavarda, D.; Trebuchon, A.; Milh, M.; Boyer, L.; Bartolomei, F.; Guedj, E. Relationship between PET metabolism and SEEG epileptogenicity in focal lesional epilepsy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3130–3142. [Google Scholar] [CrossRef] [PubMed]
- Kashida, Y.; Usui, N.; Matsuda, K.; Terada, K.; Baba, K.; Kondo, A.; Hirozawa, D.; Tottori, T.; Mihara, T.; Hanaya, R.; et al. Is additional mesial temporal resection necessary for intractable epilepsy with cavernous malformations in the temporal neocortex? Epilepsy Behav. EB 2019, 92, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chassoux, F.; Rodrigo, S.; Mellerio, C.; Landré, E.; Miquel, C.; Turak, B.; Laschet, J.; Meder, J.; Roux, F.; Daumas-Duport, C.; et al. Dysembryoplastic neuroepithelial tumors: An MRI-based scheme for epilepsy surgery. Neurology 2012, 79, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Chassoux, F.; Daumas-Duport, C. Dysembryoplastic neuroepithelial tumors: Where are we now? Epilepsia 2013, 54, 129–134. [Google Scholar] [CrossRef]
- Roehri, N.; Lina, J.; Mosher, J.; Bartolomei, F.; Benar, C. Time-Frequency Strategies for Increasing High-Frequency Oscillation Detectability in Intracerebral EEG. IEEE Trans. Bio-Med. Eng. 2016, 63, 2595–2606. [Google Scholar] [CrossRef]
- Roehri, N.; Pizzo, F.; Lagarde, S.; Lambert, I.; Nica, A.; McGonigal, A.; Giusiano, B.; Bartolomei, F.; Bénar, C. High-frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann. Neurol. 2018, 83, 84–97. [Google Scholar] [CrossRef]



| Patients with Mesial Temporal LEATs | Patients with Lateral Temporal LEATs | Healthy Controls | Statistic | |
|---|---|---|---|---|
| Number | 43 | 27 | 25 | - |
| Sex (female, %) | 20 (46.5%) | 14 (51.9) | 12 (48.0%) | Pearson χ2 = 0.192, df = 2, p = 0.909 |
| Age of surgery (yrs) | 20.1 (12.0) | 23.9 (11.0) | 26.6 (8.7) * | ANOVA F = 0.2.964, df = 2, p = 0.057 |
| Duration (yrs) | 5.7 (5.2) | 7.6 (8.1) | - | Mann-Whitney U = 552.000, df = 1, p = 0.731 |
| Age of onset (yrs) | 14.4 (11.4) | 16.3 (9.5) | - | Student’s t = −0.734, df = 1, p = 0.465 |
| Side (left, %) | 18 (41.9%) | 13 (48.1%) | - | Pearson χ2 = 0.266, df = 1, p = 0.606 |
| size of LEAT (mm3) | 8253.0 (10748.6) | 6195.3 (4163.9) | - | Mann-Whitney U = 501.5, df = 1, p = 0.340 |
| Pathology (%) | GG: 33 (76.7%) DNT: 5 (11.6%) LGNETs: 2 (4.7%) AG: 1 (2.3%) PGNT: 1 (2.3%) PA: 1 (2.3%) | GG: 20 (74.1%) DNT: 5 (18.5%) AG: 1 (3.7%) LGNETs: 1 (3.7%) | - | - |
| Duration (months) | 44.1 (16.0) | 43.3 (15.0) | - | Mann-Whitney U = 555.500, p = 0.763 |
| Test for Normal Distribution | Test of Homogeneity of Variances | Within-Group Comparison | Post Hoc Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Lilliefors Statistic | p Value | Levene Statistic | p Value | Statistic | p Value | Multiple Comparisons | Contrast | MD | p Value | 95% CI | |
| PCL | Mesial | 0.103 | 0.285 | 0.146 | 0.864 | ANOVA F: 9.731 | <0.001 * | Scheffe | mesial vs. lateral | −0.007 | 0.636 | −0.024, 0.011 |
| Lateral | 0.129 | 0.289 | mesial vs. control | −0.031 | <0.001 * | −0.048, −0.013 | ||||||
| Control | 0.146 | 0.175 | latera vs. control | −0.024 | 0.010 * | −0.043, −0.005 | ||||||
| CN | Mesial | 0.090 | 0.500 | 5.062 | 0.008 * | ANOVA F: 20.749 | <0.001 * | Games-Howell | mesial vs. lateral | −0.015 | 0.017 * | −0.028, −0.002 |
| Lateral | 0.122 | 0.355 | mesial vs. control | −0.045 | <0.001 * | −0.066, −0.024 | ||||||
| Control | 0.090 | 0.500 | latera vs. control | −0.030 | 0.004 * | −0.051, −0.009 | ||||||
| Putamen | Mesial | 0.140 | 0.034 * | 3.245 | 0.043 * | Kruskal-Wallis H: 19.258 | <0.001 * | Bonferroni | mesial vs. lateral | −14.417 | 0.084 | −30.282, −1.448 |
| Lateral | 0.114 | 0.472 | mesial vs. control | −30.207 | <0.001 * | −46.457, −13.956 | ||||||
| Control | 0.152 | 0.136 | latera vs. control | −15.790 | 0.098 | −33.723, 2.144 | ||||||
| Thalamus | Mesial | 0.114 | 0.164 | 0.780 | 0.461 | ANOVA F: 4.754 | 0.011 * | Scheffe | mesial vs. lateral | −0.033 | 0.140 | −0.073, 0.008 |
| Lateral | 0.149 | 0.123 | mesial vs. control | −0.049 | 0.017 * | −0.090, −0.007 | ||||||
| Control | 0.105 | 0.500 | latera vs. control | −0.161 | 0.680 | −0.062, 0.030 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, J.; Zhang, J.; Hu, W.; Sang, L.; Shao, X.; Zhang, C.; Zhang, K. Metabolism and Intracranial Epileptogenicity in Temporal Lobe Long-Term Epilepsy-Associated Tumor. J. Clin. Med. 2022, 11, 5309. https://doi.org/10.3390/jcm11185309
Mo J, Zhang J, Hu W, Sang L, Shao X, Zhang C, Zhang K. Metabolism and Intracranial Epileptogenicity in Temporal Lobe Long-Term Epilepsy-Associated Tumor. Journal of Clinical Medicine. 2022; 11(18):5309. https://doi.org/10.3390/jcm11185309
Chicago/Turabian StyleMo, Jiajie, Jianguo Zhang, Wenhan Hu, Lin Sang, Xiaoqiu Shao, Chao Zhang, and Kai Zhang. 2022. "Metabolism and Intracranial Epileptogenicity in Temporal Lobe Long-Term Epilepsy-Associated Tumor" Journal of Clinical Medicine 11, no. 18: 5309. https://doi.org/10.3390/jcm11185309






