Maternal Right Ventricular and Left Atrial Function in Uncomplicated Twin Pregnancies: A Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Pressure Measurement
2.2. Conventional and Tissue Doppler Echocardiography
2.3. Speckle-Tracking Echocardiography
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Meah, V.L.; Cockcroft, J.R.; Backx, K.; Shave, R.; Stohr, E.J. Cardiac output and related haemodynamics during pregnancy: A series of meta-analyses. Heart 2016, 102, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, K.; Sharma, R.; Thilaganathan, B. Cardiac structure and function in normal pregnancy. Curr. Opin. Obstet. Gynecol. 2012, 24, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Kametas, N.A.; McAuliffe, F.; Krampl, E.; Chambers, J.; Nicolaides, K.H. Maternal cardiac function in twin pregnancy. Obstet. Gynecol. 2003, 102, 806–815. [Google Scholar] [CrossRef]
- Kuleva, M.; Youssef, A.; Maroni, E.; Contro, E.; Pilu, G.; Rizzo, N.; Pelusi, G.; Ghi, T. Maternal cardiac function in normal twin pregnancy: A longitudinal study. Ultrasound Obstet. Gynecol. 2011, 38, 575–580. [Google Scholar] [CrossRef]
- Ghi, T.; Kuleva, M.; Youssef, A.; Maroni, E.; Nanni, M.; Pilu, G.; Rizzo, N.; Pelusi, G. Maternal cardiac function in complicated twin pregnancy: A longitudinal study. Ultrasound Obstet. Gynecol. 2011, 38, 581–585. [Google Scholar] [CrossRef]
- Robson, S.C.; Hunter, S.; Boys, R.J.; Dunlop, W. Hemodynamic changes during twin pregnancy. A Doppler and M-mode echocardiographic study. Am. J. Obstet. Gynecol. 1989, 161, 1273–1278. [Google Scholar] [CrossRef]
- Practice Committee of American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: An American Society for Reproductive Medicine Practice Committee opinion. Fertil. Steril. 2012, 97, 825–834. [Google Scholar] [CrossRef]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369. [Google Scholar] [CrossRef]
- Orabona, R.; Sciatti, E.; Vizzardi, E.; Bonadei, I.; Metra, M.; Sartori, E.; Frusca, T.; Pinna, A.; Bellocco, R.; Prefumo, F. Maternal hemodynamics, arterial stiffness and elastic aortic properties in twin pregnancy. Physiol. Meas. 2021, 41, 125001. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M.; et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur. J. Echocardiogr. 2011, 12, 167–205. [Google Scholar] [CrossRef]
- Orabona, R.; Vizzardi, E.; Sciatti, E.; Bonadei, I.; Valcamonico, A.; Metra, M.; Frusca, T. Insights into cardiac alterations after pre-eclampsia: An echocardiographic study. Ultrasound Obstet. Gynecol. 2017, 49, 124–133. [Google Scholar] [CrossRef]
- Cameli, M.; Caputo, M.; Mondillo, S.; Ballo, P.; Palmerini, E.; Lisi, M.; Marino, E.; Galderisi, M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound 2009, 7, 6. [Google Scholar] [CrossRef]
- Kurt, M.; Wang, J.; Torre-Amione, G.; Nagueh, S.F. Left atrial function in diastolic heart failure. Circ. Cardiovasc. Imaging 2009, 2, 10–15. [Google Scholar] [CrossRef]
- Geraci, M. Linear Quantile Mixed Models: The lqmm Package for Laplace Quantile Regression. J. Stat. Softw. 2014, 57, 1–29. [Google Scholar] [CrossRef]
- Duvekot, J.J.; Cheriex, E.C.; Pieters, F.A.; Peeters, L.L. Severely impaired fetal growth is preceded by maternal hemodynamic maladaptation in very early pregnancy. Acta Obstet. Gynecol. Scand. 1995, 74, 693–697. [Google Scholar] [CrossRef]
- Vasapollo, B.; Valensise, H.; Novelli, G.P.; Altomare, F.; Galante, A.; Arduini, D. Abnormal maternal cardiac function precedes the clinical manifestation of fetal growth restriction. Ultrasound Obstet. Gynecol. 2004, 24, 23–29. [Google Scholar] [CrossRef]
- De Paco, C.; Kametas, N.; Rencoret, G.; Strobl, I.; Nicolaides, K.H. Maternal cardiac output between 11 and 13 weeks of gestation in the prediction of preeclampsia and small for gestational age. Obstet. Gynecol. 2008, 111, 292–300. [Google Scholar] [CrossRef]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right Ventricular Function in Cardiovascular Disease, Part II. Pathophysiology, Clinical Importance, and Management of Right Ventricular Failure. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, J.; Snyder, E.M.; Hassager, C.; Oh, J.K.; Johnson, B.D. Impact of preload and afterload on global and regional right ventricular function and pressure: A quantitative echocardiography study. J. Am. Soc. Echocardiogr. 2006, 19, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Sciatti, E.; Vizzardi, E.; Bonadei, I.; Curnis, A.; D’Aloia, A.; Metra, M. Prognostic value of RV isovolumic acceleration and tissue strain in moderate HFrEF. Eur. J. Clin. Investig. 2015, 45, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Hunter, S.; Robson, S.C. Adaptation of the maternal heart in pregnancy. Br. Heart J. 1992, 68, 540–543. [Google Scholar] [CrossRef]
- Ghi, T.; Dall’Asta, A.; Franchi, L.; Fieni, S.; Gaibazzi, N.; Siniscalchi, C.; Pedrazzi, G.; Montaguti, E.; Degli Esposti, D.; Carpano, M.G.; et al. The Effect of Chorionicity on Maternal Cardiac Adaptation to Uncomplicated Twin Pregnancy: A Prospective Longitudinal Study. Fetal Diagn. Ther. 2019, 45, 394–402. [Google Scholar] [CrossRef]
- Grobman, W.A.; Bailit, J.L.; Rice, M.M.; Wapner, R.J.; Reddy, U.M.; Varner, M.W.; Thorp JMJr Leveno, K.J.; Caritis, S.N.; Iams, J.D.; Tita, A.T.; et al. Frequency of and factors associated with severe maternal morbidity. Obstet. Gynecol. 2014, 123, 804–810. [Google Scholar] [CrossRef]
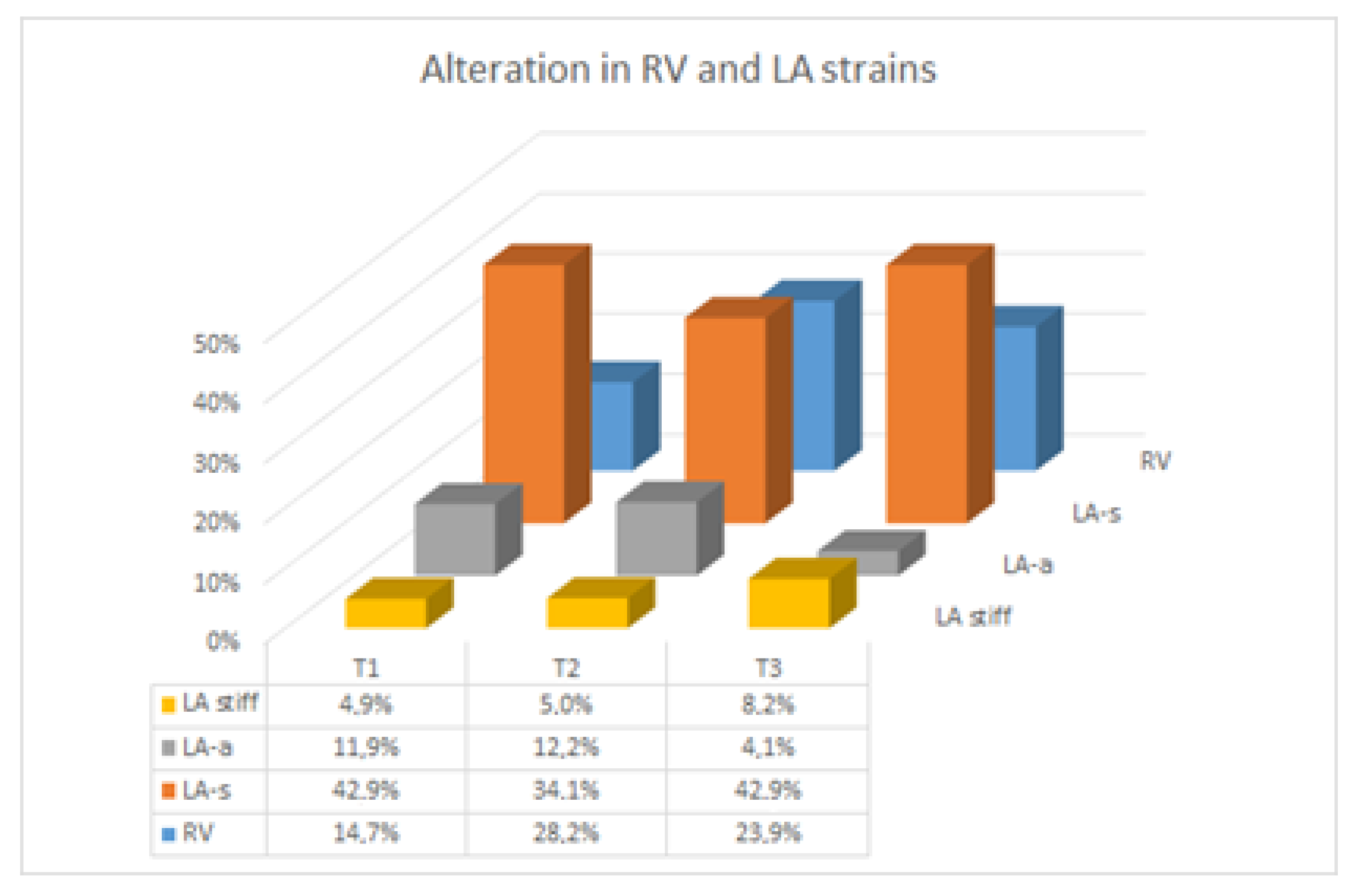
| 1st | 2nd | 3rd | p (Trend) | Longitudinal Model | Twin Dipendency | ||
|---|---|---|---|---|---|---|---|
| FAC (%) | 44.6 ± 8.3 | 46.8 ± 8.8 | 40.5 ± 11.0 | 0.2467 | p | no | |
| 46.9 ± 7.0 | 45.6 ± 6.9 | 44.0 ± 8.2 | 0.1589 | Twin | |||
| 0.2754 | 0.587 | 0.2329 | Singleton | ||||
| TAPSE (mm) | 25 ± 5 | 28 ± 4 | 26 ± 4 | 0.8534 | p | no | |
| 25 ± 4 | 27 ± 5 | 27 ± 5 | 0.0285 | Twin | |||
| 0.8192 | 0.2204 | 0.1518 | Singleton | ||||
| sPAP (mmHg) | 21 ± 3 | 20 ± 2 | 21 ± 3 | 0.1486 | p | no | |
| 21 ± 4 | 21 ± 3 | 23 ± 4 | 0.1596 | Twin | |||
| 0.7456 | 0.3882 | 0.4992 | Singleton | ||||
| E (m/s) | 0.57 ± 0.17 | 0.61± 0.21 | 0.53 ± 0.14 | 0.6226 | p | no | |
| 0.66 ± 0.17 | 0.61 ± 0.10 | 0.60 ± 0.12 | 0.0974 | Twin | |||
| 0.07286 | 0.9182 | 0.07409 | Singleton | ||||
| A (m/s) | 0.38 ± 0.12 | 0.44± 0.24 | 0.34 ± 0.13 | 0.4962 | p | no | |
| 0.40 ± 0.14 | 0.38 ± 0.09 | 0.38 ± 0.10 | 0.8756 | Twin | |||
| 0.5959 | 0.2234 | 0.1828 | Singleton | ||||
| E/A | 1.53 ± 0.29 | 1.47 ± 0.32 | 1.71 ± 0.73 | 0.3374 | p | no | |
| 1.72 ± 0.42 | 1.68 ± 0.41 | 1.62 ± 0.37 | 0.3302 | Twin | |||
| 0.06618 | 0.05839 | 0.6102 | Singleton | ||||
| DT (ms) | 173 ± 29 | 172 ± 14 | 174 ± 21 | 0.9539 | p | no | |
| 177 ± 21 | 176 ± 17 | 184 ± 25 | 0.2312 | Twin | |||
| 0.5353 | 0.3116 | 0.1246 | Singleton | ||||
| E’ (m/s) | −0.178 ± 0.043 | −0.191 ± 0.041 | −0.158 ± 0.032 | 0.0028 | p | yes, linear | no |
| −0.186 ± 0.036 | −0.185 ± 0.036 | −0.162 ± 0.033 | 0.0033 | Twin | |||
| 0.4327 | 0.5361 | 0.7052 | Singleton | ||||
| A’ (m/s) | −0.118 ± 0.073 | −0.159 ± 0.046 | −0.151 ± 0.056 | 0.0359 | p | yes, linear | no |
| −0.129 ± 0.060 | −0.138 ± 0.037 | −0.173 ± 0.061 | 0.0060 | Twin | |||
| 0.5275 | 0.07303 | 0.1869 | Singleton | ||||
| S’ basal (m/s) | 0.154 ± 0.025 | 0.183 ± 0.034 | 0.167 ± 0.034 | 0.0338 | p | yes, linear no | |
| 0.167 ± 0.026 | 0.164 ± 0.027 | 0.175 ± 0.032 | 0.0382 | Twin | |||
| 0.0552 | 0.02291 | 0.3972 | Singleton | ||||
| S’ midwall (m/s) | 0.124 ± 0.025 | 0.129 ± 0.024 | 0.5058 | 0.124 ± 0.025 | p | no | |
| 0.131 ± 0.029 | 0.128 ± 0.027 | 0.633 | 0.131 ± 0.029 | Twin | |||
| 0.123 ± 0.034 | 0.139 ± 0.051 | 0.1655 | 0.123 ± 0.034 | Singleton | |||
| S’ apical (m/s) | 0.076 ± 0.020 | 0.089 ± 0.025 | 0.080 ± 0.025 | 0.3395 | p | no | |
| 0.080 ± 0.023 | 0.080 ± 0.029 | 0.087 ± 0.033 | 0.1880 | Twin | |||
| 0.4946 | 0.2242 | 0.3754 | Singleton | ||||
| E’/A’ | 1.16 ± 1.11 | 1.31 ± 0.47 | 1.18 ± 0.46 | 0.0111 (**) | p | no | |
| 1.31 ± 0.77 | 1.41 ± 0.36 | 1.04 ± 0.39 | 0.8307 | Twin | |||
| 0.5635 | 0.3619 | 0.2552 | Singleton | ||||
| E/E’ | 3.4 ± 1.1 | 3.3 ± 1.2 | 3.3 ± 0.7 | NA | p | no | |
| 3.6 ± 1.3 | 3.2 ± 1.3 | 3.9 ± 1.3 | 0.4203 | Twin | |||
| 0.4923 | 0.6703 | 0.08289 | Singleton | ||||
| IVA (m/s2) | 4.2 ± 1.6 | 4.1 ± 1.3 | 4.1 ± 1.6 | 0.7039 | p | yes, linear | yes |
| 3.6 ± 0.8 | 4.2 ± 1.5 | 5.2 ± 2.6 | 0.0042 | Twin | |||
| 0.06295 | 0.8148 | 0.07895 | Singleton | ||||
| IVCT (ms) | 66 ± 17 | 61 ± 18 | 65 ± 20 | 0.7107 | p | no | |
| 75 ± 20 | 65 ± 15 | 68 ± 19 | 0.1472 | Twin | |||
| 0.08391 | 0.368 | 0.5175 | Singleton | ||||
| IVRT (ms) | 54 ± 24 | 53 ± 25 | 44 ± 12 | 0.0088 | p | yes, linear | yes |
| 57 ± 21 | 52 ± 17 | 46 ± 15 | 0.1010 | Twin | |||
| 0.6104 | 0.9262 | 0.7177 | Singleton | ||||
| ET (ms) | 282 ± 21 | 275 ± 29 | 265 ± 31 | 0.0071 | p | no | |
| 276 ± 31 | 271 ± 29 | 258 ± 31 | 0.0048 | Twin | |||
| 0.4529 | 0.6007 | 0.4357 | Singleton | ||||
| MPI | 0.43 ± 0.13 | 0.42 ± 0.16 | 0.42 ± 0.11 | 0.7731 | p | no | |
| 0.48 ± 0.13 | 0.44 ± 0.12 | 0.45 ± 0.11 | NA | Twin | |||
| 0.1138 | 0.645 | 0.3105 | Singleton | ||||
| Longitudinal 2D strain (%) | −23.3 ± 5.4 | −20.1 ± 3.6 | −22.8 ± 4.3 | 0.8905 | p | no | |
| −21.0 ± 3.1 | −20.9 ± 2.7 | −20.7 ± 3.9 | 0.7041 | Twin | |||
| 0.1495 | 0.4422 | 0.1074 | Singleton | ||||
| 1st | 2nd | 3rd | p (Trend) | Longitudinal Model | Twin Dipendency | ||
|---|---|---|---|---|---|---|---|
| Antero-posterior diameter (mm) | 31 ± 4 | 35 ± 3 | 38 ± 3 | <0.00009 | p | yes, linear | no |
| 32 ± 5 | 35 ± 4 | 37 ± 5 | <0.00009 | Twin | |||
| 0.3284 | 0.9875 | 0.4128 | Singleton | ||||
| Area (cm2) | 12.8 ± 2.8 | 15.5 ± 2.7 | 15.4 ± 3.3 | 0.0001 | p | yes, linear | no |
| 13.8 ± 3.3 | 16.0 ± 3.0 | 17.1 ± 6.5 | 0.0018 | Twin | |||
| 0.1999 | 0.473 | 0.2292 | Singleton | ||||
| Volume (mL) | 27 ± 9 | 34 ± 10 | 36 ± 9 | <0.00009 (***) | p | yes, linear | no |
| 26 ± 9 | 36 ± 15 | 36 ± 11 | <0.00009 (**) | Twin | |||
| 0.7512 | 0.6751 | 0.9893 | Singleton | ||||
| Volume index (mL/m2) | 15.7 ± 5.3 | 19.4 ± 5.8 | 19.2 ± 5.2 | <0.00009 (**) | p | yes, linear | no |
| 14.7 ± 5.5 | 20.3 ± 7.8 | 19.9 ± 5.5 | <0.00009 (**) | Twin | |||
| 0.4985 | 0.6336 | 0.6737 | Singleton | ||||
| LAS 2D strain (%) | 36.5 ± 19.8 | 33.8 ± 12.7 | 32.1 ± 12.4 | 0.2073 | p | no | |
| 30.8 ± 11.4 | 38.2 ± 15.5 | 32.0 ± 14.3 | 0.9814 | Twin | |||
| 0.2497 | 0.33 | 0.981 | Singleton | ||||
| LAA 2D strain (%) | 12.2 ± 7.0 | 10.6 ± 4.7 | 12.4 ± 5.2 | 0.9402 | p | no | |
| 8.6 ± 3.9 | 12.8 ± 7.6 | 11.4 ± 5.6 | 0.1429 | Twin | |||
| 0.04893 | 0.2808 | 0.523 | Singleton | ||||
| Stiffness | 0.18 ± 0.11 | 0.20 ± 0.10 | 0.21 ± 0.10 | 0.15653 | Twin | no | |
| 0.19 ± 0.10 | 0.16 ± 0.11 | 0.22 ± 0.12 | 0.2857 | Singleton | |||
| 0.7445 | 0.1893 | 0.8271 | p | ||||
| Base Value | β (Twin) | β (Time) | β (Time2) | Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff. | p | Coeff. | p | Coeff. | p | Coeff. | p | p | |
| BMI | 22.60 ± 0.46 | 0.00 | 0.15 ± 0.13 | 0.26 | 0.45 ± 0.04 | <0.0009 | <0.00009 | ||
| BSA | 1.69 ± 0.02 | 0.00 | 0.006 ± 0.004 | 0.123 | 0.013 ± 0.01 | <0.0009 | <0.00009 | ||
| E’ | −0.1871947 ± 0.0049126 | 0.00 | 0.0104935 ± 0.0032543 | 0.001 | 0.0013 | ||||
| A’ | −0.1248308 ± 0.0076612 | 0.00 | −0.0205315 ± 0.0059499 | 0.001 | 0.0006 | ||||
| S’ basal | 0.1626717 ± 0.0031544 | 0.00 | 0.00608 ± 0.0023773 | 0.011 | 0.0105 | ||||
| IVA (**) | 3.550077 ± 0.2406082 | 0.00 | 0.6719929 ± 0.3383214 | 0.047 | 0.7676086 ± 0.238221 | 0.001 | 0.0142 | ||
| IVRT | 55.77271 ± 2.688193 | 0.00 | −4.921733 ± 1.708916 | 0.004 | 0.0040 | ||||
| ET | 279.7668 ± 3.277997 | 0.00 | −8.860039 ± 2.244868 | <0.0009 | 0.0001 | ||||
| LA antero−posterior diameter | 32.01954 ± 0.5196185 | 0.00 | 2.821833 ± 0.2300515 | <0.0009 | <0.00009 | ||||
| LA area | 13.56488 ± 0.3788698 | 0.00 | 1.532933 ± 0.3241711 | <0.0009 | <0.00009 | ||||
| LA volume | 26.53448 ± 1.316003 | 0.00 | 12.37174 ± 2.05069 | <0.0009 | −3.975581 ± 0.9950033 | <0.0009 | <0.00009 | ||
| LA volume index | 15.1887 ± 0.7612453 | 0.00 | 6.876214 ± 1.167011 | <0.0009 | −2.45863 ± 0.5667266 | <0.0009 | <0.00009 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orabona, R.; Sciatti, E.; Vizzardi, E.; Bonadei, I.; Metra, M.; Sartori, E.; Frusca, T.; Pinna, A.; Bellocco, R.; Prefumo, F. Maternal Right Ventricular and Left Atrial Function in Uncomplicated Twin Pregnancies: A Longitudinal Study. J. Clin. Med. 2022, 11, 5432. https://doi.org/10.3390/jcm11185432
Orabona R, Sciatti E, Vizzardi E, Bonadei I, Metra M, Sartori E, Frusca T, Pinna A, Bellocco R, Prefumo F. Maternal Right Ventricular and Left Atrial Function in Uncomplicated Twin Pregnancies: A Longitudinal Study. Journal of Clinical Medicine. 2022; 11(18):5432. https://doi.org/10.3390/jcm11185432
Chicago/Turabian StyleOrabona, Rossana, Edoardo Sciatti, Enrico Vizzardi, Ivano Bonadei, Marco Metra, Enrico Sartori, Tiziana Frusca, Antonio Pinna, Rino Bellocco, and Federico Prefumo. 2022. "Maternal Right Ventricular and Left Atrial Function in Uncomplicated Twin Pregnancies: A Longitudinal Study" Journal of Clinical Medicine 11, no. 18: 5432. https://doi.org/10.3390/jcm11185432
APA StyleOrabona, R., Sciatti, E., Vizzardi, E., Bonadei, I., Metra, M., Sartori, E., Frusca, T., Pinna, A., Bellocco, R., & Prefumo, F. (2022). Maternal Right Ventricular and Left Atrial Function in Uncomplicated Twin Pregnancies: A Longitudinal Study. Journal of Clinical Medicine, 11(18), 5432. https://doi.org/10.3390/jcm11185432





