A Different rTMS Protocol for a Different Type of Depression: 20.000 rTMS Pulses for the Treatment of Bipolar Depression Type II
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Study Sample Subgroups
2.3. Procedure of rTMS
2.4. BDI and SCL-90-R Questionnaires
2.5. Statistical Procedure
3. Results
3.1. Tolerability
3.2. Clinical Efficacy
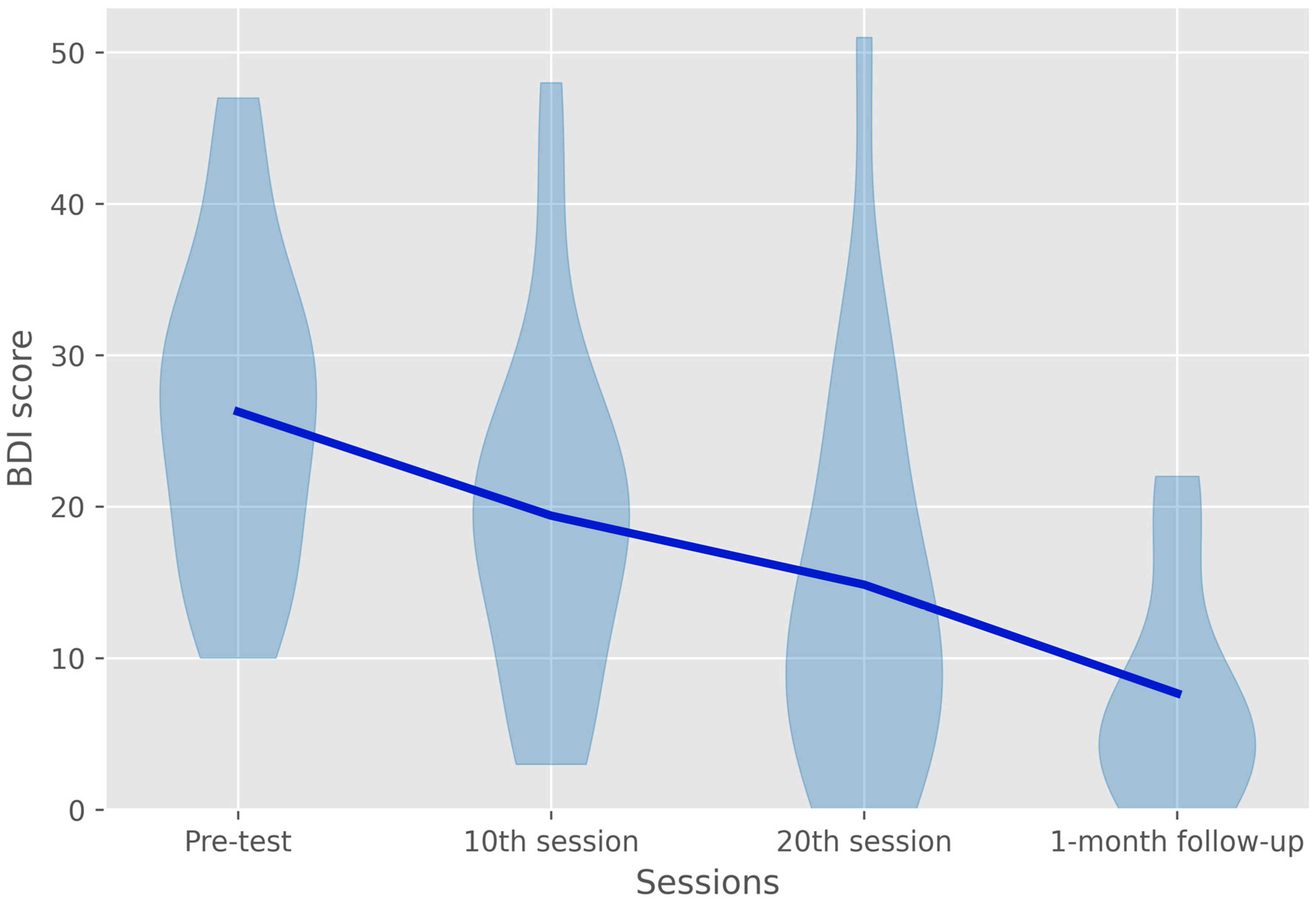
3.3. Beck Depression Inventory
3.4. Symptom Checklist-90-Revised Depression Subscale
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fagiolini, A.; Forgione, R.; Maccari, M.; Cuomo, A.; Morana, B.; Dell’Osso, M.C.; Pellegrini, F.; Rossi, A. Prevalence, chronicity, burden and borders of bipolar disorder. J. Affect. Disord. 2013, 148, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Harnic, D.; Gonda, X.; Forte, A.; Dominici, G.; Innamorati, M.; Fountoulakis, K.N.; Serafini, G.; Sher, L.; Janiri, L.; et al. Impact of living with bipolar patients: Making sense of caregivers’ burden. World J. Psychiatry 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.; Rosenblum, K.L.; McInnis, M.G.; Muzik, M. The role of social relationships in bipolar disorder: A review. Psychiatry Res. 2014, 219, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Hermens, D.F.; Scott, J.; Redoblado-Hodge, M.A.; Naismith, S.L.; Lagopoulos, J.; Griffiths, K.R.; Porter, M.A.; Hickie, I.B. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J. Psychiatr. Res. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Jin, R.; He, J.P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and Correlates of Bipolar Spectrum Disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry 2011, 68, 241. [Google Scholar] [CrossRef]
- Weinstock, L.M.; Gaudiano, B.A.; Epstein-Lubow, G.; Tezanos, K.; Celis-deHoyos, C.E.; Miller, I.W. Medication burden in bipolar disorder: A chart review of patients at psychiatric hospital admission. Psychiatry Res. 2014, 216, 24–30. [Google Scholar] [CrossRef]
- Butler, M.; Urosevic, S.; Desai, P.; Sponheim, S.R.; Popp, J.; Nelson, V.A.; Thao, V.; Sunderlin, B. Chapter 1, Introduction. In Treatment for Bipolar Disorder in Adults: A Systematic Review [Internet]; Comparative Effectiveness Review, No. 208; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2018; pp. 1–9. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532193/ (accessed on 15 July 2022).
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Goldberg, J.F.; Freeman, M.P.; Balon, R.; Citrome, L.; Thase, M.E.; Kane, J.M.; Fava, M. The american society of clinical psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders: Research Article: Mood Disorders Prescribing Survey. Depress. Anxiety 2015, 32, 605–613. [Google Scholar] [CrossRef]
- Ghaemi, S.N. Treatment of Rapid-Cycling Bipolar Disorder: Are Antidepressants Mood Destabilizers? Am. J. Psychiatry 2008, 165, 300–302. [Google Scholar] [CrossRef]
- Pilhatsch, M.; Stamm, T.J.; Stahl, P.; Lewitzka, U.; Berghöfer, A.; Sauer, C.; Gitlin, M.; Frye, M.A.; Whybrow, P.C.; Bauer, M. Treatment of bipolar depression with supraphysiologic doses of levothyroxine: A randomized, placebo-controlled study of comorbid anxiety symptoms. Int. J. Bipolar Disord. 2019, 7, 21. [Google Scholar] [CrossRef]
- Andy, Z.; Robin, R.; Alexander, B.-A.; Abbi, L.; Christos, K. High-Dose Levothyroxine for Bipolar Disorder; the Potential Role of Thyroid Function and Genetic Tests. Report from Twenty Cases. Int. J. Psychiatry Res. 2021, 4, 1–7. [Google Scholar] [CrossRef]
- Suttajit, S.; Srisurapanont, M.; Maneeton, N.; Maneeton, B. Quetiapine for acute bipolar depression: A systematic review and meta-analysis. Drug Des Devel. Ther. 2014, 8, 827. [Google Scholar] [CrossRef]
- Koutsomitros, T.; Evagorou, O.; Schuhmann, T.; Zamar, A.; Sack, A.T. Advances in transcranial magnetic stimulation (TMS) and its applications in resistant depression. Psychiatriki 2021, 32 (Suppl. S1), 90–98. [Google Scholar] [CrossRef]
- Cohen, S.L.; Bikson, M.; Badran, B.W.; George, M.S. A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices. Brain Stimul. 2022, 15, 73–75. [Google Scholar] [CrossRef]
- Bermudes, R.A.; Lanocha, K.I.; Janicak, P.G. (Eds.) Transcranial Magnetic Stimulation: Clinical Applications for Psychiatric Practice, 1st ed.; American Psychiatric Association Publishing: Arlington, VA, USA, 2018; 216p. [Google Scholar]
- Milev, R.V.; Giacobbe, P.; Kennedy, S.H.; Blumberger, D.M.; Daskalakis, Z.J.; Downar, J.; Modirrousta, M.; Patry, S.; Vila-Rodriguez, F.; Lam, R.W.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 4. Neurostimulation Treatments. Can. J. Psychiatry 2016, 61, 561–575. [Google Scholar] [CrossRef]
- Taylor, D.M.; Barnes, T.R.E.; Young, A.H. The Maudsley Prescribing Guidelines in Psychiatry, 14th ed.; The maudsley prescribing guidelines series; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Schlaepfer, T.E.; George, M.S.; Helen Mayberg on behalf of the WFSBP Task Force on Brain Stimulation. WFSBP Guidelines on Brain Stimulation Treatments in Psychiatry. World J. Biol. Psychiatry 2010, 11, 2–18. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial Magnetic Stimulation: A Primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Valls-Solé, J.; Wassermann, E.M.; Hallett, M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994, 117, 847–858. [Google Scholar] [CrossRef]
- Baeken, C.; Brem, A.K.; Arns, M.; Brunoni, A.R.; Filipčić, I.; Ganho-Ávila, A.; Langguth, B.; Padberg, F.; Poulet, E.; Rachid, F.; et al. Repetitive transcranial magnetic stimulation treatment for depressive disorders: Current knowledge and future directions. Curr. Opin. Psychiatry 2019, 32, 409–415. [Google Scholar] [CrossRef]
- Cohen, D.A.; Freitas, C.; Tormos, J.M.; Oberman, L.; Eldaief, M.; Pascual-Leone, A. Enhancing plasticity through repeated rTMS sessions: The benefits of a night of sleep. Clin. Neurophysiol. 2010, 121, 2159–2164. [Google Scholar] [CrossRef] [Green Version]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Chaimani, A.; Moffa, A.H.; Razza, L.B.; Gattaz, W.F.; Daskalakis, Z.J.; Carvalho, A.F. Repetitive Transcranial Magnetic Stimulation for the Acute Treatment of Major Depressive Episodes: A Systematic Review with Network Meta-analysis. JAMA Psychiatry 2017, 74, 143. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.K.; Ornelas, A.C.; Cirillo, P.; Caldieraro, M.A.; Nardi, A.E.; Nierenberg, A.A.; Kinrys, G. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019, 9, e01419. Available online: https://onlinelibrary.wiley.com/doi/10.1002/brb3.1419 (accessed on 13 July 2022). [CrossRef] [PubMed]
- McGirr, A.; Karmani, S.; Arsappa, R.; Berlim, M.T.; Thirthalli, J.; Muralidharan, K.; Yatham, L.N. Clinical efficacy and safety of repetitive transcranial magnetic stimulation in acute bipolar depression. World Psychiatry 2016, 15, 85–86. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Hieronymus, F.; Lorentzen, R.; McGirr, A.; Østergaard, S.D. The efficacy of repetitive transcranial magnetic stimulation (rTMS) for bipolar depression: A systematic review and meta-analysis. J. Affect. Disord. 2020, 279, 250–255. [Google Scholar] [CrossRef]
- Judd, L.L.; Akiskal, H.S.; Schettler, P.J.; Endicott, J.; Maser, J.; Solomon, D.A.; Leon, A.C.; Rice, J.A.; Keller, M.B. The Long-term Natural History of the Weekly Symptomatic Status of Bipolar I Disorder. Arch. Gen. Psychiatry 2002, 59, 530–537. [Google Scholar] [CrossRef]
- Forte, A.; Baldessarini, R.J.; Tondo, L.; Vázquez, G.H.; Pompili, M.; Girardi, P. Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J. Affect. Disord. 2015, 178, 71–78. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Hoy, K.E.; Elliot, D.; McQueen, S.; Wambeek, L.E.; Daskalakis, Z.J. A negative double-blind controlled trial of sequential bilateral rTMS in the treatment of bipolar depression. J. Affect. Disord. 2016, 198, 158–162. [Google Scholar] [CrossRef]
- Rachid, F. Repetitive Transcranial Magnetic Stimulation and Treatment-emergent Mania and Hypomania: A Review of the Literature. J. Psychiatr. Pract. 2017, 23, 150–159. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Maller, J.J.; Thomson, L.; Mulsant, B.H.; Rajji, T.K.; Maher, M.; Brown, P.E.; Downar, J.; Vila-Rodriguez, F.; Fitzgerald, P.B.; et al. Unilateral and bilateral MRI-targeted repetitive transcranial magnetic stimulation for treatment- resistant depression: A randomized controlled study. J. Psychiatry Neurosci. 2016, 41, E58–E66. [Google Scholar] [CrossRef] [Green Version]
- Herwig, U.; Cardenas-Morales, L.; Connemann, B.J.; Kammer, T.; Schönfeldt-Lecuona, C. Sham or real--post hoc estimation of stimulation condition in a randomized transcranial magnetic stimulation trial. Neurosci. Lett. 2010, 471, 30–33. [Google Scholar] [CrossRef]
- Herwig, U.; Fallgatter, A.J.; Höppner, J.; Eschweiler, G.W.; Kron, M.; Hajak, G.; Padberg, F.; Naderi-Heiden, A.; Abler, B.; Eichhammer, P.; et al. Antidepressant effects of augmentative transcranial magnetic stimulation: Randomised multicentre trial. Br. J. Psychiatry 2007, 191, 441–448. [Google Scholar] [CrossRef]
- Sayar, G.H.; Ozten, E.; Tan, O.; Tarhan, N. Transcranial magnetic stimulation for treating depression in elderly patients. Neuropsychiatr. Dis. Treat. 2013, 9, 501–504. [Google Scholar] [CrossRef]
- Sayar, G.H.; Ozten, E.; Tufan, E.; Cerit, C.; Kağan, G.; Dilbaz, N.; Tarhan, N. Transcranial magnetic stimulation during pregnancy. Arch. Women’s Ment. Health 2013, 17, 311–315. [Google Scholar] [CrossRef]
- Wideman, T.H.; Sullivan, M.J.L.; Inada, S.; McIntyre, D.; Kumagai, M.; Yahagi, N.; Turner, J.R.; Upton, J.; Burns, R.J.; Rothman, A.J.; et al. Beck Depression Inventory (BDI). In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 178–179. Available online: http://link.springer.com/10.1007/978-1-4419-1005-9_441 (accessed on 10 July 2022).
- Richter, P.; Werner, J.; Heerlein, A.; Kraus, A.; Sauer, H. On the Validity of the Beck Depression Inventory. Psychopathology 1998, 31, 160–168. [Google Scholar] [CrossRef]
- Richter, P. Zur Konstruktvalidität des Beck-Depressionsinventars (BDI) bei der Erfassung Depressiver Verläufe: Ein Empirischer und Methodischer Beitrag (Psychologie); Theorie und Forschung; Roderer Verlag: Regensburg, Germany, 1991; 306p. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Carbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Derogatis, L. SCL-90-R: SCL 90 R Administration, Scoring and Procedures Manual II for the Revised Version and Other Instruments of the Psychopathology Rating Scale Series; Clinical Psychometric Research: Towson, MD, USA, 1992. [Google Scholar]
- Holi, M. Assessment of Psychiatric Symptoms Using the SCL-90. Ph.D. Thesis, Department of Psychiatry, Helsinki University, Helsinki, Finland, 2003. [Google Scholar]
- Mann, J.J. The Medical Management of Depression. N. Engl. J. Med. 2005, 353, 1819–1834. [Google Scholar] [CrossRef]
- Gershon, A.A.; Dannon, P.N.; Grunhaus, L. Transcranial Magnetic Stimulation in the Treatment of Depression. Am. J. Psychiatry 2003, 160, 835–845. [Google Scholar] [CrossRef]
- Üçok, A.; Karaveli, D.; Kundakçi, T.; Yazici, O. Comorbidity of personality disorders with bipolar mood disorders. Compr. Psychiatry 1998, 39, 72–74. [Google Scholar] [CrossRef]
- Altshuler, L.; Suppes, T.; Black, D.; Nolen, W.A.; Keck, P.E.; Frye, M.A.; McElroy, S.; Kupka, R.; Grunze, H.; Walden, J.; et al. Impact of Antidepressant Discontinuation after Acute Bipolar Depression Remission on Rates of Depressive Relapse at 1-Year Follow-Up. Am. J. Psychiatry 2003, 160, 1252–1262. [Google Scholar] [CrossRef] [Green Version]
- Hunter, A.M.; Minzenberg, M.J.; Cook, I.A.; Krantz, D.E.; Levitt, J.G.; Rotstein, N.M.; Chawla, S.; Leuchter, A.F. Concomitant medication use and clinical outcome of repetitive Transcranial Magnetic Stimulation (rTMS) treatment of Major Depressive Disorder. Brain Behav. 2019, 9, e01275. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.B.; Yip, A.; Siddiqui, R.; Morales, O.G.; Seiner, S.J.; Siddiqi, S.H. Borderline personality traits do not influence response to TMS. J. Affect. Disord. 2020, 281, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Nassan, M.; Singh, B.; Croarkin, P.E.; Kung, S. Response rate underreports improvement in patients with major depressive disorder and comorbidities treated with repetitive transcranial magnetic stimulation (rTMS). Psychiatry Res. Commun. 2022, 2, 100033. [Google Scholar] [CrossRef]
- Gündoğmuş, İ.; Örnek, B.; Altınyay, N. The Impact of Repetetive Transcranial Magnetic Stimulation (rTMS) Treatment on Borderline Personality Disorder Symptoms: A Case Report. Psychiatry Behav. Sci. 2019, 9, 53–56. [Google Scholar] [CrossRef]

| Any Personality Disorder | Any Benzodiazepines | Total | ||
|---|---|---|---|---|
| No | Yes | |||
| No | 7 | 3 | 10 | |
| Females | 4 | 0 | 4 | |
| Males | 3 | 3 | 6 | |
| Yes | 5 | 8 | 13 | |
| Females | 1 | 7 | 8 | |
| Males | 4 | 1 | 5 | |
| Total | 12 | 11 | 23 | |
| Females | 5 | 7 | 12 | |
| Males | 7 | 4 | 11 | |
| Parameter | Numerator df | Denominator df | F-Statistic | p-Value |
|---|---|---|---|---|
| Session | 3 | 35.99 | 0.717 | 0.549 |
| Any personality disorder | 1 | 31.002 | 0.002 | 0.964 |
| Benzodiazepine use | 1 | 31.918 | 0.346 | 0.561 |
| Bidaily treatment | 1 | 19.059 | 0.153 | 0.7 |
| Age | 1 | 32.467 | 0.478 | 0.494 |
| Pre-test | 1 | 22.468 | 9.566 | 0.005 |
| Session * Any personality disorder | 3 | 35.564 | 0.08 | 0.97 |
| Session * Benzodiazepine use | 3 | 35.632 | 0.58 | 0.632 |
| Session * Bidaily treatment | 3 | 36 | 0.576 | 0.634 |
| Session * Age | 3 | 36.52 | 0.576 | 0.634 |
| Session * Pre-test | 3 | 35.938 | 6.187 | 0.002 |
| Session | Mean Difference | df | p-Value | |
|---|---|---|---|---|
| Pre-test | 10th session | 7.176 | 33.028 | 0.009 |
| 20th session | 10.982 | 34.776 | <0.001 | |
| Follow-up | 18.187 | 38.873 | <0.001 | |
| 10th session | Pre-test | −7.176 | 33.028 | 0.009 |
| 20th session | 3.806 | 34.776 | 0.614 | |
| Follow-up | 11.011 | 38.873 | 0.050 | |
| 20th session | Pre-test | −10.982 | 34.776 | <0.001 |
| 10th session | −3.806 | 34.776 | 0.614 | |
| Follow-up | 7.205 | 38.789 | 0.497 | |
| Follow-up | Pre-test | −18.187 | 38.873 | <0.001 |
| 10th session | −11.011 | 38.873 | 0.050 | |
| 20th session | −7.205 | 38.789 | 0.497 | |
| Parameter | Numerator df | Denominator df | F-Statistic | p-Value |
|---|---|---|---|---|
| Session | 3 | 35.975 | 1.723 | 0.180 |
| Any personality disorder | 1 | 33.678 | 1.978 | 0.169 |
| Benzodiazepine use | 1 | 33.317 | 0.807 | 0.375 |
| Bidaily treatment | 1 | 18.855 | 0.000 | 0.995 |
| Age | 1 | 32.737 | 0.184 | 0.671 |
| Pre-test | 1 | 26.764 | 6.476 | 0.017 |
| Session * Any personality disorder | 3 | 36.199 | 0.963 | 0.420 |
| Session * Benzodiazepine use | 3 | 36.324 | 0.813 | 0.495 |
| Session * Bidaily treatment | 3 | 35.890 | 0.284 | 0.837 |
| Session * Age | 3 | 37.194 | 0.096 | 0.962 |
| Session * Pre-test | 3 | 36.695 | 5.309 | 0.004 |
| Session | Mean Difference | df | p-Value | |
|---|---|---|---|---|
| Pre-test | 10th session | 5.322 | 33.441 | 0.099 |
| 20th session | 9.814 | 35.353 | <0.001 | |
| Follow-up | 15.607 | 39.647 | 0.002 | |
| 10th session | Pre-test | −5.322 | 33.441 | 0.099 |
| 20th session | 4.492 | 35.353 | 0.355 | |
| Follow-up | 10.285 | 39.647 | 0.076 | |
| 20th session | Pre-test | −9.814 | 35.353 | <0.001 |
| 10th session | −4.492 | 35.353 | 0.355 | |
| Follow-up | 5.793 | 39.776 | 0.959 | |
| Follow-up | Pre-test | −15.607 | 39.647 | 0.002 |
| 10th session | −10.285 | 39.647 | 0.076 | |
| 20th session | −5.793 | 39.776 | 0.959 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsomitros, T.; van der Zee, K.T.; Evagorou, O.; Schuhmann, T.; Zamar, A.C.; Sack, A.T. A Different rTMS Protocol for a Different Type of Depression: 20.000 rTMS Pulses for the Treatment of Bipolar Depression Type II. J. Clin. Med. 2022, 11, 5434. https://doi.org/10.3390/jcm11185434
Koutsomitros T, van der Zee KT, Evagorou O, Schuhmann T, Zamar AC, Sack AT. A Different rTMS Protocol for a Different Type of Depression: 20.000 rTMS Pulses for the Treatment of Bipolar Depression Type II. Journal of Clinical Medicine. 2022; 11(18):5434. https://doi.org/10.3390/jcm11185434
Chicago/Turabian StyleKoutsomitros, Theodoros, Kenneth T. van der Zee, Olympia Evagorou, Teresa Schuhmann, Antonis C. Zamar, and Alexander T. Sack. 2022. "A Different rTMS Protocol for a Different Type of Depression: 20.000 rTMS Pulses for the Treatment of Bipolar Depression Type II" Journal of Clinical Medicine 11, no. 18: 5434. https://doi.org/10.3390/jcm11185434
APA StyleKoutsomitros, T., van der Zee, K. T., Evagorou, O., Schuhmann, T., Zamar, A. C., & Sack, A. T. (2022). A Different rTMS Protocol for a Different Type of Depression: 20.000 rTMS Pulses for the Treatment of Bipolar Depression Type II. Journal of Clinical Medicine, 11(18), 5434. https://doi.org/10.3390/jcm11185434







