Knee Kinematic Patterns and Early Cartilage Lesion Characteristics in Patients with Anterior Cruciate Ligament Reconstruction
Abstract
1. Introduction
2. Materials and Methods
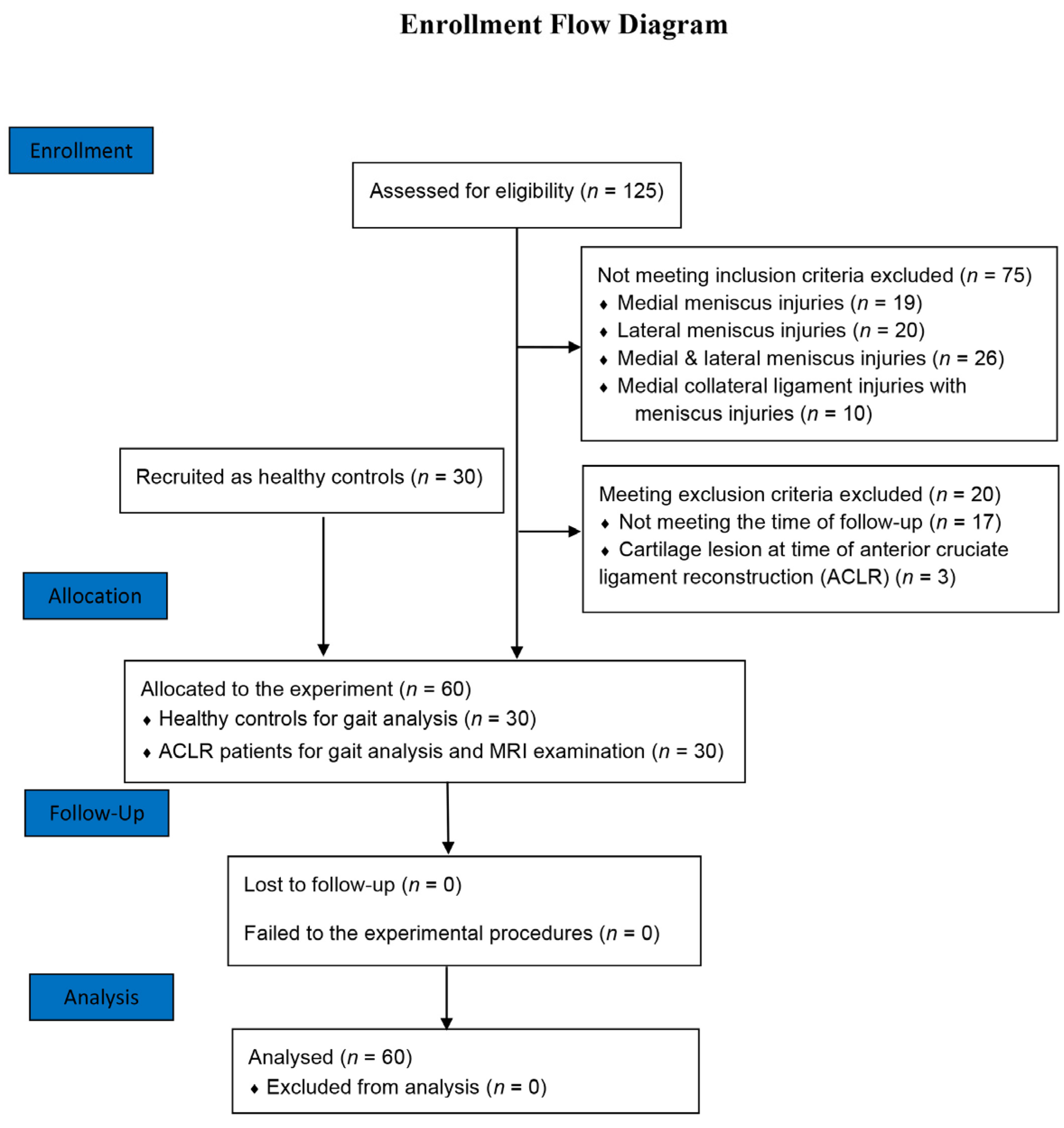
2.1. Subjects
2.2. Magnetic Resonance Imaging (MRI) Examination
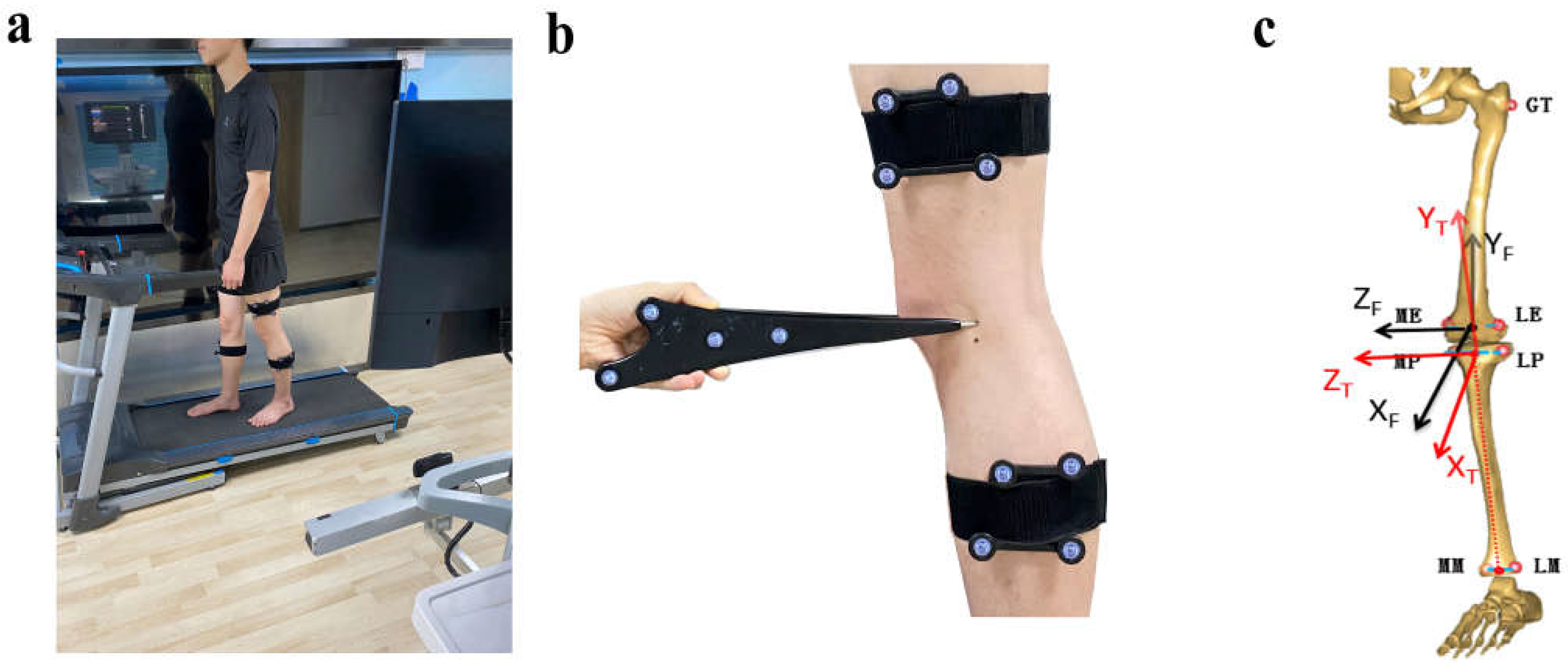
2.3. Kinematic Alerations of ACLR Knees during Walking
2.4. Clinical Score Assessment
2.5. Statistical Analysis
3. Results
3.1. The Setup of Kinematic Characteristics and Patterns during the Stance Phase
3.2. CL Distribution Characteristics Supports Kinematic Patterns to Contribute to Specific Cartilage Areas of CL Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Svantesson, E.; Senorski, E.H.; Webster, K.E.; Karlsson, J.; Diermeier, T.; Rothrauff, B.B.; Meredith, S.J.; Rauer, T.; Irrgang, J.J.; Spindler, K.P.; et al. Clinical outcomes after anterior cruciate ligament injury: Panther symposium ACL injury clinical outcomes consensus group. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2415–2434. [Google Scholar] [CrossRef] [PubMed]
- Ajuied, A.; Wong, F.; Smith, C.; Norris, M.; Earnshaw, P.; Back, D.; Davies, A. Anterior Cruciate Ligament Injury and Radiologic Progression of Knee Osteoarthritis: A systematic review and meta-analysis. Am. J. Sports Med. 2014, 42, 2242–2252. [Google Scholar] [CrossRef] [PubMed]
- Haughom, B.; Schairer, W.; Souza, R.B.; Carpenter, D.; Ma, C.B.; Li, X. Abnormal tibiofemoral kinematics following ACL reconstruction are associated with early cartilage matrix degeneration measured by MRI T1rho. Knee 2012, 19, 482–487. [Google Scholar] [CrossRef]
- Teng, H.-L.; Wu, D.; Su, F.; Pedoia, V.; Souza, R.B.; Ma, C.B.; Li, X. Gait Characteristics Associated with a Greater Increase in Medial Knee Cartilage T1ρ and T2 Relaxation Times in Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2017, 45, 3262–3271. [Google Scholar] [CrossRef]
- Williams, A.A.; Titchenal, M.R.; Andriacchi, T.P.; Chu, C.R. MRI UTE-T2* profile characteristics correlate to walking mechanics and patient reported outcomes 2 years after ACL reconstruction. Osteoarthr. Cartil. 2018, 26, 569–579. [Google Scholar] [CrossRef]
- Zampeli, F.; Pappas, E.; Velonakis, G.; Roumpelakis, I.M.; Poulou, L.S.; Papagiannis, G.I.; Kelekis, A.D.; Mastrokalos, D.S. Development of new cartilage lesions after ACL reconstruction is associated with abnormal knee rotation. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.M.W.; Briant, P.L.; Bevill, S.L.; Koo, S.; Andriacchi, T.P. Knee Kinematics, Cartilage Morphology, and Osteoarthritis after ACL Injury. Med. Sci. Sports Exerc. 2008, 40, 215–222. [Google Scholar] [CrossRef]
- Stergiou, N.; Ristanis, S.; Moraiti, C.O.; Georgoulis, A.D. Tibial Rotation in Anterior Cruciate Ligament (ACL)-Deficient and ACL-Reconstructed Knees: A theoretical proposition for the development of osteoarthritis. Sports Med. 2007, 37, 601–613. [Google Scholar] [CrossRef]
- Koo, S.; Rylander, J.H.; Andriacchi, T.P. Knee joint kinematics during walking influences the spatial cartilage thickness distribution in the knee. J. Biomech. 2011, 44, 1405–1409. [Google Scholar] [CrossRef]
- Gold, G.E.; Chen, C.A.; Koo, S.; Hargreaves, B.A.; Bangerter, N.K. Recent Advances in MRI of Articular Cartilage. AJR Am. J. Roentgenol. 2009, 193, 628–638. [Google Scholar] [CrossRef]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.J.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.-P.; Raynauld, J.-P.; Berthiaume, M.-J.; Abram, F.; Choquette, D.; Haraoui, B.; Beary, J.F.; Cline, G.A.; Meyer, J.M.; Martel-Pelletier, J. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: A longitudinal study. Arthritis Res. Ther. 2007, 9, R74. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, J.-S.; Torriani, M.; Hosseini, A. Short-Term Contact Kinematic Changes and Longer-Term Biochemical Changes in the Cartilage After ACL Reconstruction: A Pilot Study. Ann. Biomed. Eng. 2018, 46, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Citation-classic—A coefficient of agreement for nominal scales. Curr. Contents/Soc. Behav. Sci. 1986, 3, 18. [Google Scholar]
- Zhang, Y.; Yao, Z.; Wang, S.; Huang, W.; Ma, L.; Huang, H.; Xia, H. Motion analysis of Chinese normal knees during gait based on a novel portable system. Gait Posture 2015, 41, 763–768. [Google Scholar] [CrossRef]
- Elfring, R.; De La Fuente, M.; Radermacher, K. Assessment of optical localizer accuracy for computer aided surgery systems. Comput. Aided Surg. 2010, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, W.-H.; Yao, Z.; Ma, L.; Lin, Z.; Wang, S.; Huang, H. Anterior Cruciate Ligament Injuries Alter the Kinematics of Knees with or without Meniscal Deficiency. Am. J. Sports Med. 2016, 44, 3132–3139. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Richards, J.; Whittle, M.W. (Eds.) Whittle’s Gait Analysis, 5th ed.; Churchill Livingstone/Elsevier: Edinburgh, UK; New York, NY, USA, 2012. [Google Scholar]
- Perry, J.; Burnfield, J.M. Gait Analysis: Normal and Pathological Function, 2nd ed.; SLACK: Thorofare, NJ, USA, 2010. [Google Scholar]
- Lertwanich, P.; Praphruetkit, T.; Keyurapan, E.; Lamsam, C.; Kulthanan, T. Validity and reliability of Thai version of the International Knee Documentation Committee Subjective Knee Form. J. Med. Assoc. Thail. 2008, 91, 1218–1225. [Google Scholar]
- Briggs, K.K.; Lysholm, J.; Tegner, Y.; Rodkey, W.G.; Kocher, M.S.; Steadman, J.R. The Reliability, Validity, and Responsiveness of the Lysholm Score and Tegner Activity Scale for Anterior Cruciate Ligament Injuries of the Knee: 25 years later. Am. J. Sports Med. 2009, 37, 890–897. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman & Hall/CRC: Boca Raton, FL, USA, 1999. [Google Scholar]
- Moglo, K.; Shirazi-Adl, A. Cruciate coupling and screw-home mechanism in passive knee joint during extension–flexion. J. Biomech. 2005, 38, 1075–1083. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Dyrby, C.O. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J. Biomech. 2005, 38, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Beard, D.J.; Murray, D.W.; Gill, H.; Price, A.J.; Rees, J.; Alfaro-Adrian, J.; Dodd, C.A.F. Reconstruction does not reduce tibial translation in the cruciate-deficient knee: An in vivo study. J. Bone Jt. Surg. Br. Vol. 2001, 83, 1098–1103. [Google Scholar] [CrossRef]
- Gao, B.; Zheng, N. Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin. Biomech. 2010, 25, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, A.D.; Papadonikolakis, A.; Papageorgiou, C.D.; Mitsou, A.; Stergiou, N. Three-Dimensional Tibiofemoral Kinematics of the Anterior Cruciate Ligament-Deficient and Reconstructed Knee during Walking. Am. J. Sports Med. 2003, 31, 75–79. [Google Scholar] [CrossRef]
- Chmielewski, T.L.; Ramsey, D.K.; Snyder-Mackler, L. Evidence for differential control of tibial position in perturbed unilateral stance after acute ACL rupture. J. Orthop. Res. 2005, 23, 54–60. [Google Scholar] [CrossRef]
- Zaid, M.; Lansdown, D.A.; Su, F.; Pedoia, V.; Tufts, L.; Rizzo, S.; Souza, R.B.; Li, X.; Ma, C.B. Abnormal tibial position is correlated to early degenerative changes one year following ACL reconstruction. J. Orthop. Res. 2015, 33, 1079–1086. [Google Scholar] [CrossRef]
- Ikuta, F.; Yoneta, K.; Miyaji, T.; Kidera, K.; Yonekura, A.; Osaki, M.; Gamada, K. Knee kinematics of severe medial knee osteoarthritis showed tibial posterior translation and external rotation: A cross-sectional study. Aging Clin. Exp. Res. 2020, 32, 1767–1775. [Google Scholar] [CrossRef]
- Kiapour, A.M.; Fleming, B.C.; Murray, M.M. Structural and Anatomic Restoration of the Anterior Cruciate Ligament Is Associated with Less Cartilage Damage 1 Year After Surgery: Healing Ligament Properties Affect Cartilage Damage. Orthop. J. Sports Med. 2017, 5, 2325967117723886. [Google Scholar] [CrossRef]
- Li, A.K.; Ochoa, J.K.; Pedoia, V.; Amano, K.; Souza, R.B.; Li, X.; Ma, C.B. Altered tibiofemoral position following ACL reconstruction is associated with cartilage matrix changes: A voxel-based relaxometry analysis. J. Orthop. Res. 2020, 38, 2454–2463. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Mündermann, A.; Smith, R.L.; Alexander, E.J.; Dyrby, C.O.; Koo, S. A Framework for the in Vivo Pathomechanics of Osteoarthritis at the Knee. Ann. Biomed. Eng. 2004, 32, 447–457. [Google Scholar] [CrossRef]
- Besier, T.F.; Fredericson, M.; Gold, G.E.; Beaupre, G.; Delp, S.L. Knee muscle forces during walking and running in patellofemoral pain patients and pain-free controls. J. Biomech. 2009, 42, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Myers, S.L.; Burr, D.; Albrecht, M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Care Res. 1991, 34, 1560–1570. [Google Scholar] [CrossRef]
- Li, G.; Moses, J.M.; Papannagari, R.; Pathare, N.P.; DeFrate, L.E.; Gill, T.J. Anterior Cruciate Ligament Deficiency Alters the In Vivo Motion of the Tibiofemoral Cartilage Contact Points in Both the Anteroposterior and Mediolateral Directions. J. Bone Jt. Surg. 2006, 88, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, J.A.; Graham, G.P.; Dent, C.M. Radiological sign of chronic anterior cruciate ligament deficiency. Injury 1990, 21, 401–402. [Google Scholar] [CrossRef]
- Frank, C.B.; Beveridge, J.E.; Huebner, K.D.; Heard, B.J.; Tapper, J.E.; O’Brien, E.J.O.; Shrive, N.G. Complete ACL/MCL deficiency induces variable degrees of instability in sheep with specific kinematic abnormalities correlating with degrees of early osteoarthritis. J. Orthop. Res. 2012, 30, 384–392. [Google Scholar] [CrossRef]
- Sharma, L.; Song, J.; Felson, D.T.; Cahue, S.; Shamiyeh, E.; Dunlop, D.D. The Role of Knee Alignment in Disease Progression and Functional Decline in Knee Osteoarthritis. JAMA 2001, 286, 188–195. [Google Scholar] [CrossRef] [PubMed]
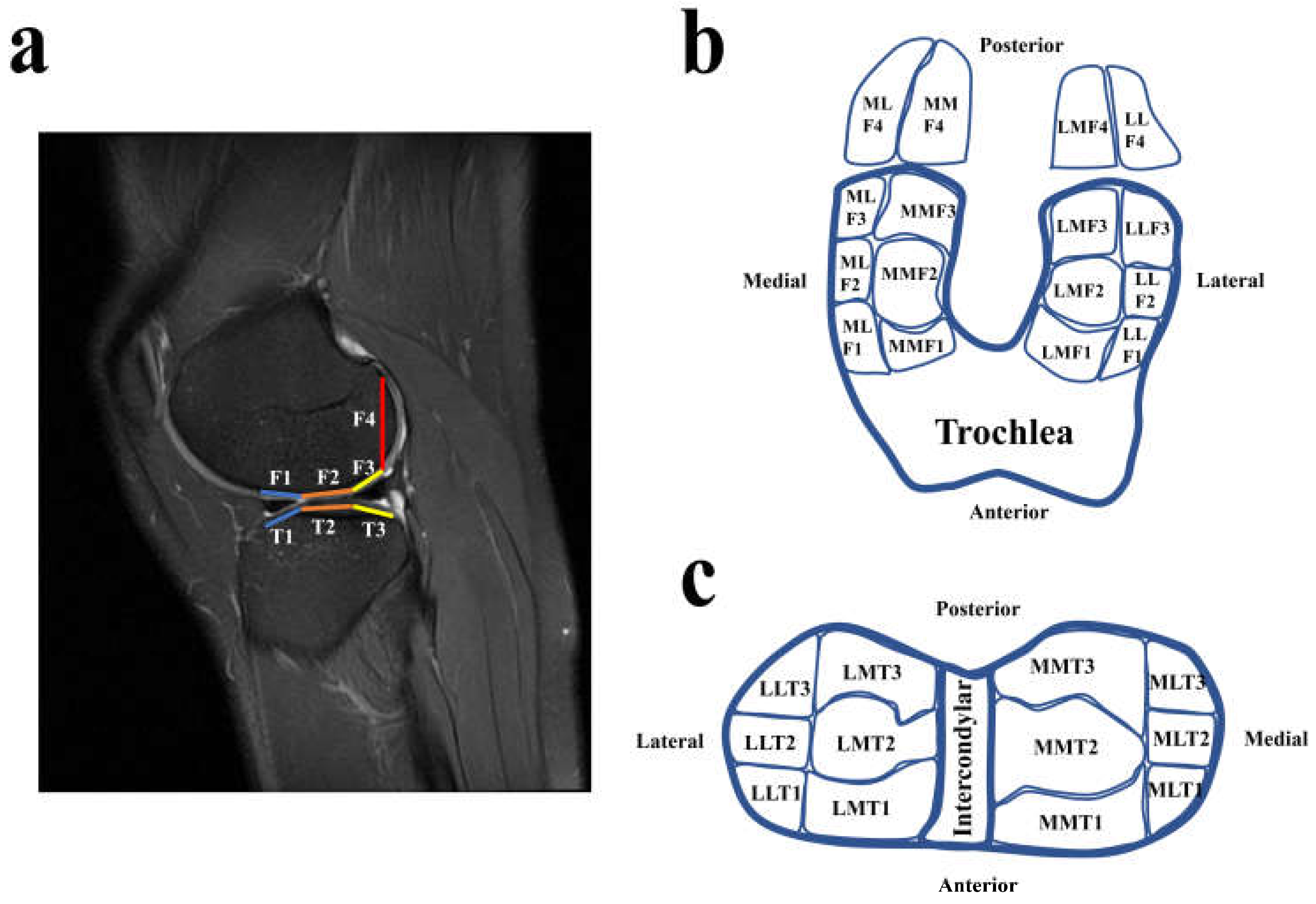

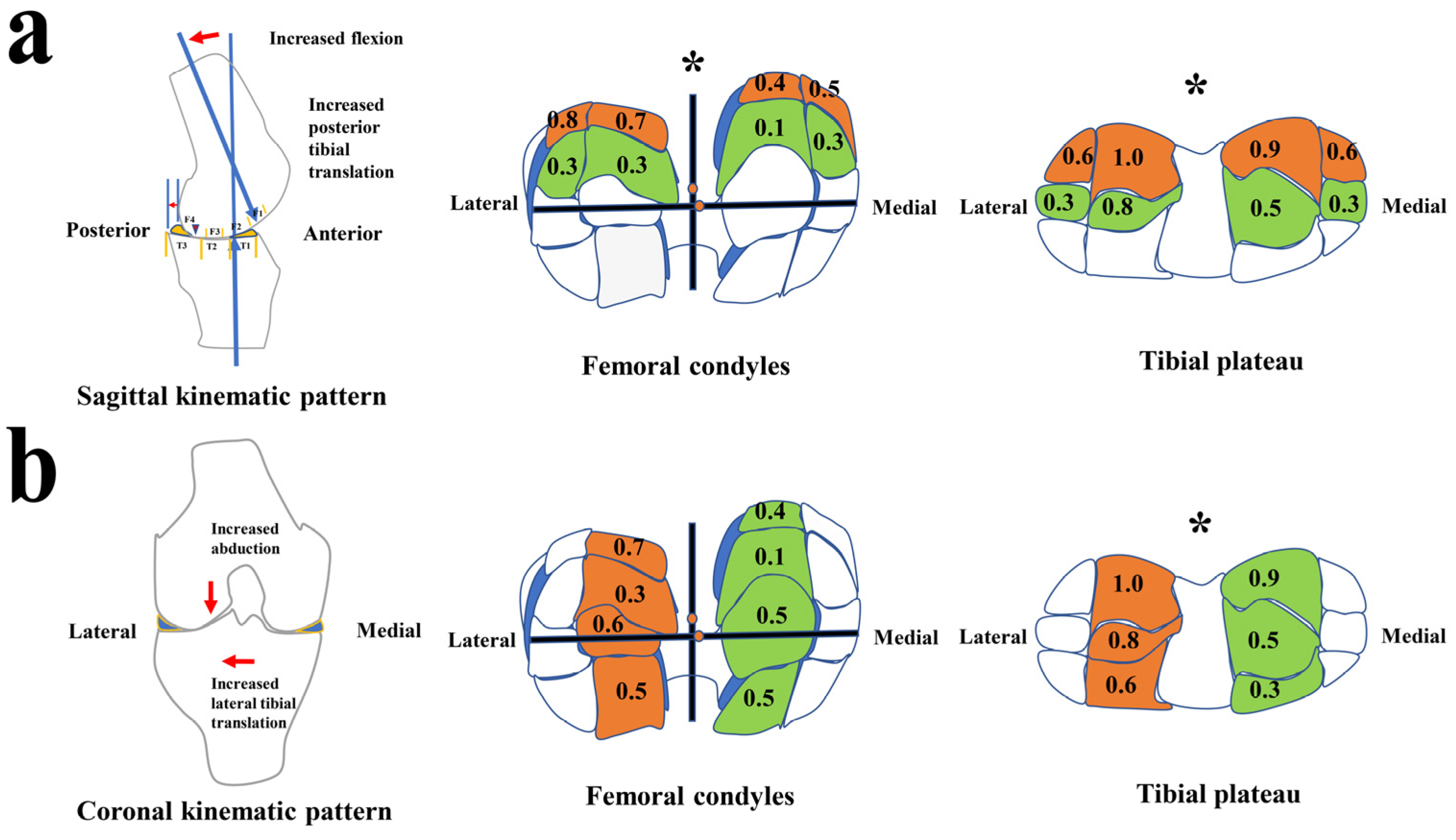
| Medial Compartments (Medial Side) | Cartilage Lesions (%) | Mean | Medial Compartments (Lateral Side) | Cartilage Lesions (%) | Mean | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | 0 | I | II | III | IV | ||||||
| Anterior | MMF1 | 19 | 7 | 4 | - | - | 0.5 | Anterior | MLF1 | 11 | 11 | 8 | - | - | 0.9 |
| Middle | MMF2 | 17 | 11 | 2 | - | - | 0.5 | Middle | MLF2 | 12 | 13 | 5 | - | - | 0.8 |
| Posterior | MMF3 | 26 | 4 | - | - | - | 0.1 | Posterior | MLF3 | 23 | 5 | 2 | - | - | 0.3 |
| Superoposterior | MMF4 | 19 | 9 | 2 | - | - | 0.4 | Superoposterior | MLF4 | 20 | 7 | 2 | 1 | - | 0.5 |
| Anterior | MMT1 | 20 | 10 | - | - | - | 0.3 | Anterior | MLT1 | 21 | 7 | 2 | - | - | 0.4 |
| Middle | MMT2 | 18 | 11 | 1 | - | - | 0.5 | Middle | MLT2 | 20 | 10 | - | - | - | 0.3 |
| Posterior | MMT3 | 13 | 9 | 7 | - | - | 0.9 | Posterior | MLT3 | 17 | 7 | 6 | - | - | 0.6 |
| Lateral compartments (medial side) | Lateral compartments (lateral side) | ||||||||||||||
| Anterior | LMF1 | 18 | 8 | 4 | - | - | 0.5 | Anterior | LLF1 | 19 | 8 | 3 | - | - | 0.5 |
| Middle | LMF2 | 17 | 9 | 4 | - | - | 0.6 | Middle | LLF2 | 16 | 9 | 3 | 1 | 1 | 0.7 |
| Posterior | LMF3 | 22 | 7 | 1 | - | - | 0.3 | Posterior | LLF3 | 22 | 7 | 1 | - | - | 0.3 |
| Superoposterior | LMF4 | 16 | 8 | 6 | - | - | 0.7 | Superoposterior | LLF4 | 11 | 14 | 5 | - | - | 0.8 |
| Anterior | LMT1 | 14 | 13 | 2 | 3 | - | 0.6 | Anterior | LLT1 | 21 | 6 | 3 | - | - | 0.4 |
| Middle | LMT2 | 7 | 21 | 2 | - | - | 0.8 | Middle | LLT2 | 21 | 8 | 1 | - | - | 0.3 |
| Posterior | LMT3 | 10 | 9 | 11 | - | - | 1.0 | Posterior | LLT3 | 17 | 9 | 3 | 1 | - | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Zeng, J.; Lin, J.; Kong, L.; Chen, H.; Zhong, G.; Ma, L.; Zhang, Y.; Huang, W. Knee Kinematic Patterns and Early Cartilage Lesion Characteristics in Patients with Anterior Cruciate Ligament Reconstruction. J. Clin. Med. 2022, 11, 5457. https://doi.org/10.3390/jcm11185457
Zeng X, Zeng J, Lin J, Kong L, Chen H, Zhong G, Ma L, Zhang Y, Huang W. Knee Kinematic Patterns and Early Cartilage Lesion Characteristics in Patients with Anterior Cruciate Ligament Reconstruction. Journal of Clinical Medicine. 2022; 11(18):5457. https://doi.org/10.3390/jcm11185457
Chicago/Turabian StyleZeng, Xiaolong, Jiajun Zeng, Jinpeng Lin, Lingchuang Kong, Haobin Chen, Guoqing Zhong, Limin Ma, Yu Zhang, and Wenhan Huang. 2022. "Knee Kinematic Patterns and Early Cartilage Lesion Characteristics in Patients with Anterior Cruciate Ligament Reconstruction" Journal of Clinical Medicine 11, no. 18: 5457. https://doi.org/10.3390/jcm11185457
APA StyleZeng, X., Zeng, J., Lin, J., Kong, L., Chen, H., Zhong, G., Ma, L., Zhang, Y., & Huang, W. (2022). Knee Kinematic Patterns and Early Cartilage Lesion Characteristics in Patients with Anterior Cruciate Ligament Reconstruction. Journal of Clinical Medicine, 11(18), 5457. https://doi.org/10.3390/jcm11185457






