1. Introduction
The accurate determination of renal function is essential in the routine clinical care of cancer patients [
1]. Underestimation or overestimation can lead to the over- or under-dosing of chemotherapeutic agents, inappropriate drug selection leading to therapeutic failure or the occurrence of increased treatment-related toxicity [
2].
The exact determination of renal function by inulin or radioisotope is cost-intensive, time-consuming and requires intervention. The determination of the glomerular filtration rate (GFR) by using 24 h collection (CrCL) is error-prone and requires adequate patient compliance. Equations for the estimation of GFR based on creatinine, cystatin C or both have been developed in recent decades [
3,
4]. However, serum creatinine levels may be influenced by age and muscle mass, especially in cancer patients, due to tumor-associated sarcopenia [
5].
Cystatin C is available as an additional biomarker for estimating GFR [
6], and previous studies revealed substantial intraindividual differences in GFR estimates using the cystatin C-based equation (eGFRcys) and the creatinine-based GFR equation (eGFRcr) [
7,
8]. The interpretation and clinical impact of differing eGFRs have not been clarified. The widely applied creatinine-based GFR (CKD EPIcrea) integrates age and sex but does not consider muscle mass, diet or physical activity, which may result in the over- or underestimation of renal function [
9,
10]. The National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) have recommended the increased use of cystatin C and the combined equation with creatinine and cystatin C to estimate the glomerular filtration rate [
11].
The cysteine protease inhibitor cystatin C is synthesized in all nucleated cells [
12,
13,
14], including cancer cells. It is catabolized completely in the proximal renal tubule and is not returned to circulation after glomerular filtration. For this reason, it is an ideal marker for estimating the glomerular filtration rate [
15]. However, in cancer patients, a high tumor cell burden may lead to an increased release of cystatin C into the circulation [
16,
17,
18]. LDH represents an established parameter to estimate tumor cell mass and is part of various prognosis scores [
19]. The effect of steroids and additional diseases like diabetes or systemic inflammation on cystatin C expression is also under controversy [
20,
21].
To our knowledge, no comparative studies have been performed on cancer patients in which tumor mass was also added as a confounding variable. In this study, we examined the results obtained with the different equations and the influence of tumor mass and discuss possible implications for clinical practice.
2. Methods
Serums were collected from all patients as part of routine diagnostics at the time of admission and analyzed in the laboratory of the University Medicine Göttingen. The parameters (Plasma creatinine, urine creatinine, LDH and cystatin C) were measured on the Architect c16000 device from Abbott (Abbott, Chicago, IL, USA). The study was approved by the ethics committees of the University Medical Center Göttingen (No: 19/1/22).
2.1. Serum Creatinine and 24 h Collection Urine and Equation of Estimated GFR
The measurement of serum creatinine was performed by Architect c16000 Abbott in the laboratory of the University Medical Center Göttingen. For eGFR calculation, the equation of creatinine-based (eGFRcr; 2009), the equation of cystatin-C-based (eGFRcys; 2012) and the equation of combined creatinine-cystatin C-based (eGFRcr-cys; 2012) were used [
22].
2.2. Statistics
GraphPad PRISM Version 9.1 (GraphPad Software, San Diego, CA, USA) and Statistical Package for the Social Sciences (SPSS) 27.0 (IBM Corp., Armonk, NY, USA) were used for the statistical analysis. For correlations, the two-tailed nonparametric Spearman test, two-tailed paired t-test and simple linear regression analysis were used. A one-way ANOVA Test was performed to analyze the three groups. All p-values are two-sided, the significance level is <0.05, and confidence intervals refer to 95% limits. For data documentation, Excel Version 2019 (Microsoft Software, Redmond, Washington, DC, USA) was used.
3. Results
3.1. Characteristics of the Study Participants
Biomarkers (creatinine, cystatin C and LDH) were retrospectively investigated from a total of 123 patients with an underlying hematological and oncological disease who were treated at the University Medical Center Göttingen. The mean age of the participants in this study was 59.39 ± 11.38 in a range of 24–87 years. The sex was equally distributed (male 52.8%, female 47.2%). The majority of the study participants had an NHL (Non-Hodgkin lymphoma) as their underlying disease (68.3%). In total, 77.3% of patients had a BMI in the range of healthy (18.5–24.9 kg/m
2) to overweight (25–29.9 kg/m
2). The number of underweight was only 1.6% (
Table 1).
3.2. Estimated GFR with the Use of the Three Equations
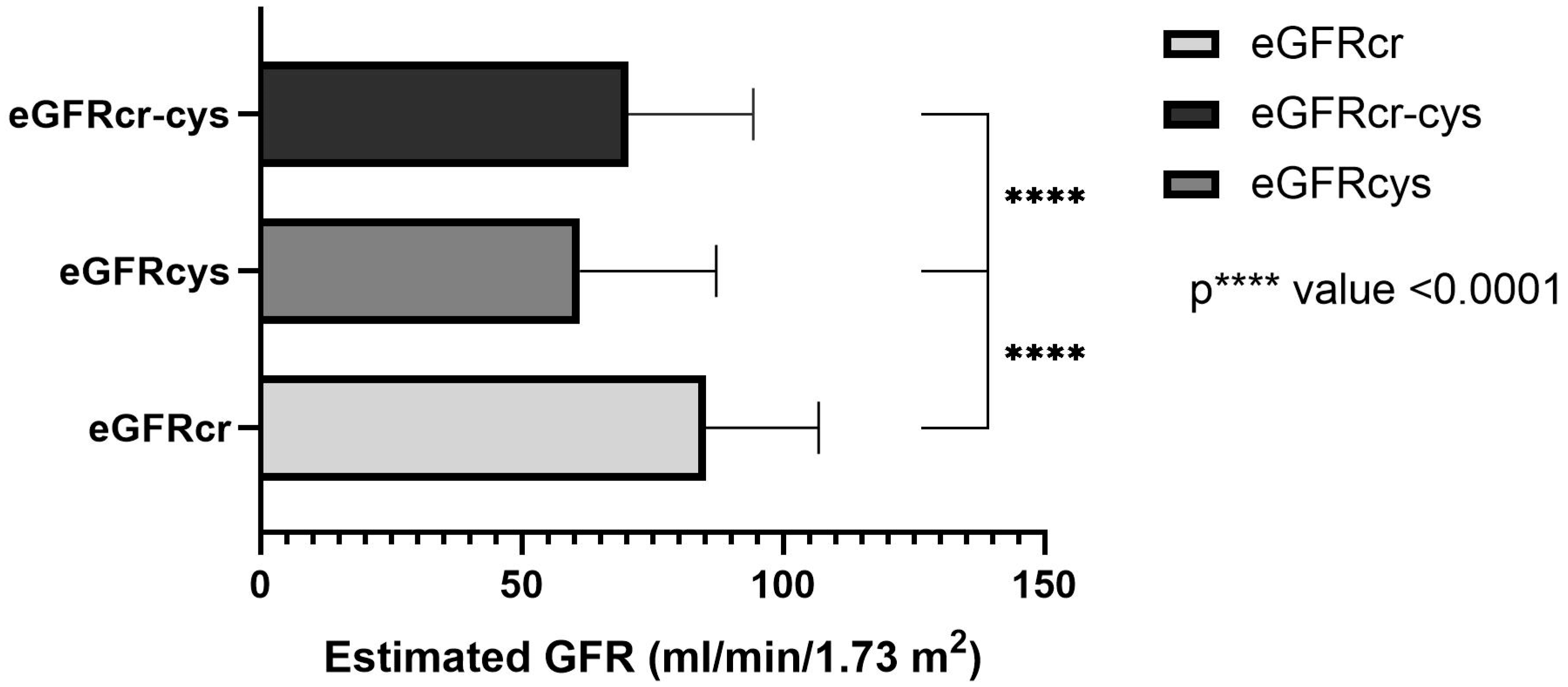
In total, the estimated GFR was determined in 123 patients based on the measured creatinine, cystatin C or both, using equations as described by Inker et al. [
21]. The mean creatinine-based equation was 85.17 ± 21.63 mL/min/1.73 m
2 and ranged between 31 and 129 mL/min/1.73 m
2. The mean cystatin C-based equation was 61.16 ± 26.03 mL/min/1.73 m
2 and ranged between 15–130 mL/min/1.73 m
2. In the combined creatinine-cystatin C-based equation, the mean was 70.42 ± 23.89 mL/min/1.73 m
2 and ranged between 23–124 mL/mL/1.73 m
2 (
Figure 1). The one-way ANOVA test shows a significant difference between all three equations (
p value < 0.0001, r = 0.147).
Visualized in the Bland–Altman-Plot, the difference in the mean values was 14.76 + 12.83 mL/min/1.73 m
2 between eGFRcr and eGFRcr-cys (
Figure 2).
Depending on the formula used, there is a different allocation of stages according to KDIGO. The difference is not significant in the one-way ANOVA test (
Figure 3).
3.3. Correlation of the Cystatin C and LDH
Correlation analysis of LDH and cystatin C was performed. Spearman’s correlation analysis between LDH and cystatin C showed a significant correlation (
p-value < 0.01, r = 0.270; n = 123) (
Figure 4).
The eGFRs based on the three equations were analyzed in relation to the plasma LDH levels. LDH values below 225 U/l (female) and 250 U/I (men) are normal and values >225 U/l (female) and >250 U/I (men) are increased. The values were divided into two groups with LDH ≤ 1.5 UNV U/l (n = 89) and >1.5 UNV (n = 34). An elevation >1.5 UNV is considered relevant and has prognostic value, e.g., in germ cell tumors. No significant difference was found using the eGFRcr. However, when using the equation eGFRcys, a significant difference was found in the Two-tailed paired
t-test between LDH ≤ 1.5 UNV and LDH >1.5 UNV U/l (
p < 0.01). Using the equation eGFRcr-cys, there was also a significant difference (
p < 0.05). In the analysis of the equations among themselves (eGFRcr vs. eGFRcys and eGFRcr vs. eGFRcr-cys), a significant difference was found in both groups (LDH ≤ 1.5 UNV n = 89;
p < 0.0001) and (LDH > 1.5 UNV n = 34;
p < 0.0001) (
Figure 5).
3.4. Subgroup Analysis of Patients with <1.5 UNV and >1.5 UNV Normal and Elevated LDH
The subgroup analysis showed an approximately equal distribution of the underlying primary disease. Parameters such as age and BMI were comparable. The number of female patients was higher in the group with LDH > UNV (n = 21; 61.8%) compared to LDH < 1.5 UNV (n = 37; 41.6%) (
Table 2).
4. Discussion
This is the first study investigating different formulas to estimate GFR in cancer patients, presenting three major findings: 1. There are significant differences in eGFR calculated by different formulas, and the use of CKD-EPIcys showed significant lower renal function compared to the estimation by CKD-EPIcrea. 2. The levels of LDH correlate with the levels of cystatin C and consecutively with eGFR levels based on CKDEPIcys. 3. In patients with elevated LDH levels above >1.5 UNV as a surrogate for high tumor mass or cell turn-over, respectively, eGFR calculated by cystatin C differs from eGFRcrea, whereas eGFR calculation in patients with LDH levels below this value did not differ using creatinine or cystatin.
Considering that cystatin C is expressed in all nucleated cells, and patients with cancer have high cell turnover, the correlation of cystatin C with LDH is of special interest [
22]. The levels of LDH are suggested to be elevated in many types of cancers and have been linked to tumor growth, and are therefore also an indication of high cell turnover [
23,
24]. In the present study, there was a significant correlation between cystatin C with LDH, suggesting an underestimation of renal function in patients with high cell turnover or tumor mass. If cell turnover and LDH levels normalize during therapy, the cystatin C-based equation would likely show higher values for eGFR compared to initial values.
By using the eGFRcys-based equation there was a significant difference in eGFR between patients with elevated LDH levels compared with patients with normal LDH levels caused by an increased level of cystatin C in patients with elevated LDH. This might contribute to the hypothesis that in the presence of increased tumor mass or high cell turnover and associated increased cystatin C, the use of the eGFRcys equation leads to an underestimation of renal function. Thus, the use of the cystatin C-based equation must be used with caution. Underestimation, in turn, is associated with underdosing of drugs, for example, and the consequent worsening of prognosis.
The non-GFR-dependent parameters, such as muscle mass, nutritional status, CRP, albumin, leukocyte count, BMI and therapy used, as well as the use of steroids, are factors that have been partially studied in the literature and significantly influence cystatin C. It should be noted that these factors have not been systematically analyzed and should be considered in further studies. Furthermore, no gold standard is used in this study as a limitation. The determination of GFR via a 24 h collection of urine was only successful in a small part of the patients and was error-prone. Therefore, no analysis can be performed. The determination of renal function in cancer patients remains difficult and each has its own difficulties. A workable gold standard that is widely used does not exist and remains to be defined. Further prospective studies are necessary, especially in patients with underlying hematological and oncological diseases. However, it could be speculated that the combination with creatinine partly compensates for this effect and that the equation with only one of these parameters may be inferior to the combination.
5. Conclusions
The present study shows a statistically significant and clinically meaningful difference in estimates of eGFR by using different eGFR equations in cancer patients. As cystatin C correlates with the levels of LDH, the evaluation of eGFRcys should be interpreted with caution in patients with elevated levels of LDH, since it might contribute to an underestimation of GFR. The use of the equation with cystatin C alone or in combination with creatinine should always be performed considering high cell turnover in cancer patients. There is a special need for further studies using the different eGFR equations to optimize the determination of kidney function.
Author Contributions
Conceptualization, E.A., M.W. and N.B.; Data curation, E.A. and N.B.; Formal analysis, E.A.; Supervision, M.W.; Writing—original draft, E.A., M.W., M.K. and N.B.; Writing—review & editing, G.G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was support by the Open Access Publication Funds of the Göttingen University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University Medical Center Göttingen (No: 19/1/22).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, V.T.D.C.E.; Costalonga, E.C.; Coelho, F.O.; Caires, R.A.; Burdmann, E. Assessment of Kidney Function in Patients with Cancer. Adv. Chronic Kidney Dis. 2018, 25, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hartlev, L.B.; Boeje, C.R.; Bluhme, H.; Palshof, T.; Rehling, M. Monitoring renal function during chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Saile, P.; Fiedler, R.; Markau, S.; Kuhn, C.; Osten, B. Bestimmung der Nierenfunktion im klinischen Alltag—Welche Methode ist die beste? [Determination of renal function in clinical routine: Which is the best method?]. Dtsch. Med. Wochenschr. (1946) 2007, 132, 1093–1097. [Google Scholar] [CrossRef]
- Delanaye, P.; Ebert, N.; Melsom, T.; Gaspari, F.; Mariat, C.; Cavalier, E.; Björk, J.; Christensson, A.; Nyman, U.; Porrini, E.; et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: A review. Part 1: How to measure glomerular filtration rate with iohexol? Clin. Kidney J. 2016, 9, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Pasala, S.; Carmody, J.B. How to use… serum creatinine, cystatin C and GFR. Arch. Dis. Child Educ. Pract. Ed. 2017, 102, 37–43. [Google Scholar] [CrossRef]
- Potok, O.A.; Katz, R.; Bansal, N.; Siscovick, D.S.; Odden, M.C.; Ix, J.H.; Shlipak, M.G.; Rifkin, D.E. The Difference Between Cystatin C- and Creatinine-Based Estimated GFR and Incident Frailty: An Analysis of the Cardiovascular Health Study (CHS). Am. J. Kidney Dis. 2020, 76, 896–898. [Google Scholar] [CrossRef]
- Potok, O.A.; Ix, J.H.; Shlipak, M.G.; Katz, R.; Hawfield, A.T.; Rocco, M.V.; Ambrosius, W.T.; Cho, M.E.; Pajewski, N.M.; Rastogi, A.; et al. The Difference Between Cystatin C- and Creatinine-Based Estimated GFR and Associations With Frailty and Adverse Outcomes: A Cohort Analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). Am. J. Kidney Dis. 2020, 76, 765–774. [Google Scholar] [CrossRef]
- Preiss, D.J.; Godber, I.M.; Lamb, E.J.; Dalton, R.N.; Gunn, I.R. The influence of a cooked-meat meal on estimated glomerular filtration rate. Ann. Clin. Biochem. 2007, 44, 35–42. [Google Scholar] [CrossRef]
- Beddhu, S.; Samore, M.H.; Roberts, M.S.; Stoddard, G.J.; Pappas, L.M.; Cheung, A.K. Creatinine production, nutrition, and glomerular filtration rate estimation. J. Am. Soc. Nephrol. 2003, 14, 1000–1005. [Google Scholar] [CrossRef] [Green Version]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. 2022, 79, 268–288.e1. [Google Scholar] [CrossRef] [PubMed]
- Onopiuk, A.; Tokarzewicz, A.; Gorodkiewicz, E. Cystatin C: A kidney function biomarker. Adv. Clin. Chem. 2015, 68, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Murty, M.S.; Sharma, U.K.; Pandey, V.B.; Kankare, S.B. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J. Nephrol. 2013, 23, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, T.; Geboes, K.; De Man, M.; Laurent, S.; Decoene, E.; Lumen, N.; Delanghe, J.; Rottey, S. Neither creatinine- nor cystatin C-estimated glomerular filtration rate is optimal in oncology patients treated with targeted agents. Nephrol. Dial. Transplant. 2018, 33, 402–408. [Google Scholar] [CrossRef]
- Ferguson, T.W.; Komenda, P.; Tangri, N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr. Opin. Nephrol. Hypertens. 2015, 24, 295–300. [Google Scholar] [CrossRef]
- Doolan, P.D.; Alpen, E.L.; Theil, G.B. A clinical appraisal of the plasma concentration and endogenous clearance of creatinine. Am. J. Med. 1962, 32, 65–79. [Google Scholar] [CrossRef]
- Nückel, H.; Langer, C.; Herget-Rosenthal, S.; Wichert, M.; Assert, R.; Döhner, H.; Dührsen, U.; Liebisch, P. Prognostic significance of serum cystatin C in multiple myeloma. Int. J. Hematol. 2012, 95, 545–550. [Google Scholar] [CrossRef]
- Hammouda, N.E.; Salah El-Din, M.A.; El-Shishtawy, M.M.; El-Gayar, A.M. Serum Cystatin C as a Biomarker in Diffuse Large B-Cell Lymphoma. Sci. Pharm. 2017, 85, 9. [Google Scholar] [CrossRef]
- Fasola, G.; Fanin, R.; Gherlinzoni, F.; Galieni, P.; Taruscio, D.; Frezza, G.; Mazza, P.; Pileri, S.; Baccarani, M. Serum LDH concentration in non-Hodgkin’s lymphomas. Relationship to histologic type, tumor mass, and presentation features. Acta Haematol. 1984, 72, 231–238. [Google Scholar] [CrossRef]
- Silva, M.V.; Moscoso Solorzano, G.; Nishida, S.K.; Kirsztajn, G.M. Are serum cystatin C levels influenced by steroid doses in lupus nephritis patients? J. Bras. Nefrol. 2011, 33, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Lean, I.; Paal, M.; Suttmann, Y.; Dengler, C.; Schönermarck, U. Comment on: ‘Effects of dose and type of corticosteroids on the divergence between estimated glomerular filtration rates derived from cystatin C and creatinine’ by Tsushita et al. J. Clin. Pharm. Ther. 2021, 46, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29, Erratum in: N. Engl. J. Med. 2012, 367, 681. Erratum in: N. Engl. J. Med. 2012, 367, 2060. [Google Scholar] [CrossRef] [PubMed]
- Pollak, J.; Szymanska, A.; Lindstrom, V.; Grubb, A. Production of Cystatin C Wild Type and Stabilized Mutants. EJIFCC 2010, 20, 166–170. [Google Scholar] [PubMed]
- Kuenzi, B.M.; Ideker, T. A census of pathway maps in cancer systems biology. Nat. Rev. Cancer 2020, 20, 233–246. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).