Determinants of Cause-Specific Mortality and Loss of Independence in Older Patients following Hospitalization for COVID-19: The GeroCovid Outcomes Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Ambulatory Follow-up
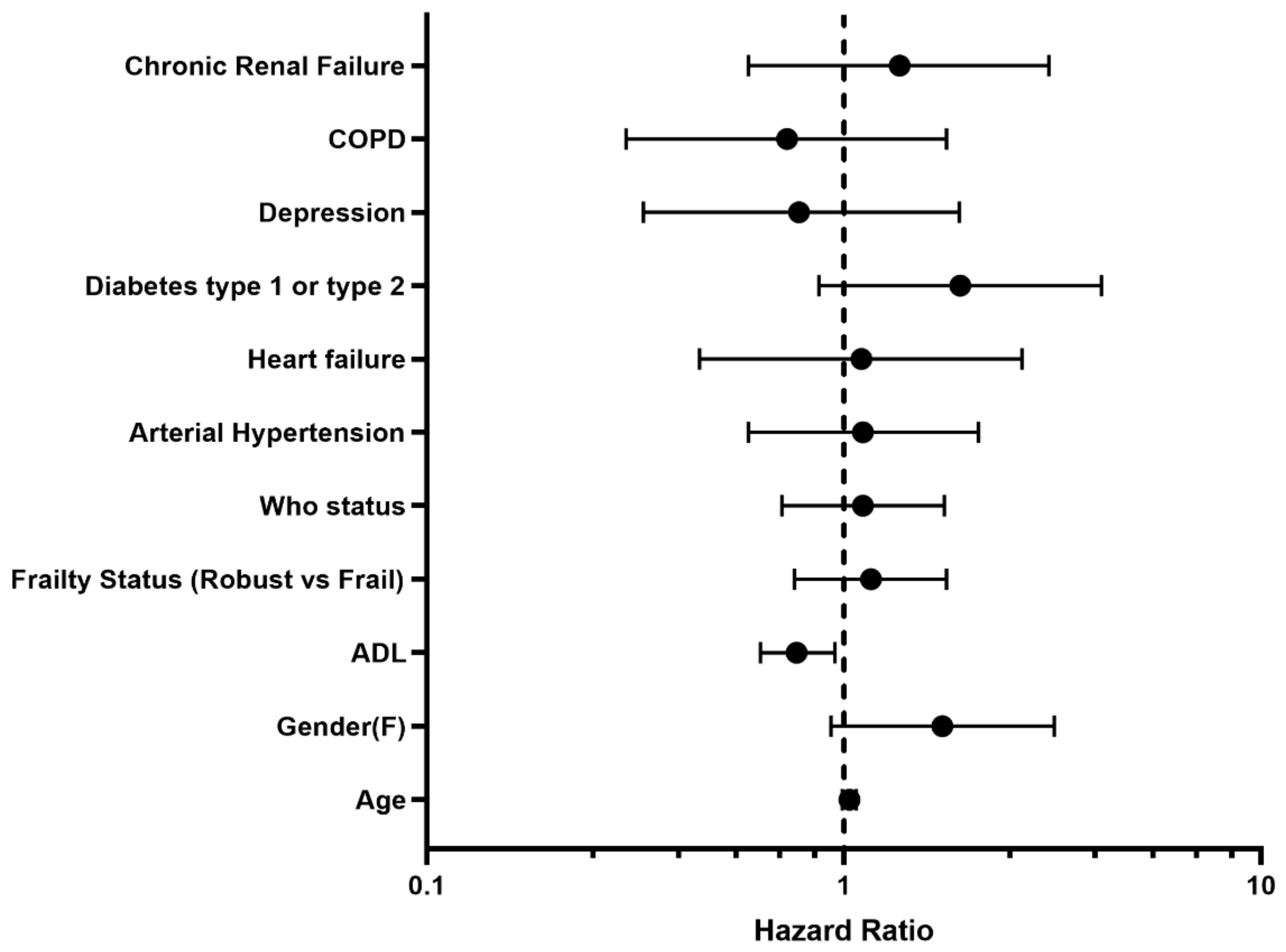
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Lai, S.; Gao, G.F.; Shi, W. The Emergence, Genomic Diversity and Global Spread of SARS-CoV-2. Nature 2021, 600, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F.; et al. New Understanding of the Damage of SARS-CoV-2 Infection Outside the Respiratory System. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef] [PubMed]
- Romero-Duarte, Á.; Rivera-Izquierdo, M.; Guerrero-Fernández de Alba, I.; Pérez-Contreras, M.; Fernández-Martínez, N.F.; Ruiz-Montero, R.; Serrano-Ortiz, Á.; González-Serna, R.O.; Salcedo-Leal, I.; Jiménez-Mejías, E.; et al. Sequelae, Persistent Symptomatology and Outcomes after COVID-19 Hospitalization: The ANCOHVID Multicentre 6-Month Follow-up Study. BMC Med. 2021, 19, 129. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Bacon, S.; Evans, S.J.; Bates, C.J.; Rentsch, C.T.; MacKenna, B.; Tomlinson, L.; Walker, A.J.; Schultze, A.; Morton, C.E.; et al. Factors Associated with Deaths Due to COVID-19 versus Other Causes: Population-Based Cohort Analysis of UK Primary Care Data and Linked National Death Registrations within the OpenSAFELY Platform. Lancet Reg. Health Eur. 2021, 6, 100109. [Google Scholar] [CrossRef]
- Günster, C.; Busse, R.; Spoden, M.; Rombey, T.; Schillinger, G.; Hoffmann, W.; Weber-Carstens, S.; Schuppert, A.; Karagiannidis, C. 6-Month Mortality and Readmissions of Hospitalized COVID-19 Patients: A Nationwide Cohort Study of 8679 Patients in Germany. PLoS ONE 2021, 16, e0255427. [Google Scholar] [CrossRef]
- Renda, G.; Ricci, F.; Spinoni, E.G.; Grisafi, L.; D’Ardes, D.; Mennuni, M.; Tana, C.; Rognoni, A.; Bellan, M.; Sainaghi, P.P.; et al. Predictors of Mortality and Cardiovascular Outcome at 6 Months after Hospitalization for COVID-19. J. Clin. Med. 2022, 11, 729. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Wang, X.Q.; Iwashyna, T.J.; Prescott, H.C. Readmission and Death After Initial Hospital Discharge Among Patients With COVID-19 in a Large Multihospital System. JAMA 2021, 325, 304–306. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Palmer, R.M.; Fortinsky, R.H.; Counsell, S.R.; Stewart, A.L.; Kresevic, D.; Burant, C.J.; Landefeld, C.S. Loss of Independence in Activities of Daily Living in Older Adults Hospitalized with Medical Illnesses: Increased Vulnerability with Age. J. Am. Geriatr. Soc. 2003, 51, 451–458. [Google Scholar] [CrossRef]
- Gómez-Uranga, A.; Guzmán-Martínez, J.; Esteve-Atiénzar, P.J.; Wikman-Jorgensen, P.; Núñez-Cruz, J.M.; Espinosa-Del-Barrio, L.; Hernández-Isasi, I.; Pomares-Gómez, F.J.; Perelló-Camacho, E.; Fernández-García, N.; et al. Nutritional and Functional Impact of Acute SARS-CoV-2 Infection in Hospitalized Patients. J. Clin. Med. 2022, 11, 2424. [Google Scholar] [CrossRef]
- Lyu, W.; Wolinsky, F.D. The Onset of ADL Difficulties and Changes in Health-Related Quality of Life. Health Qual. Life Outcomes 2017, 15, 217. [Google Scholar] [CrossRef] [Green Version]
- Sleiman, I.; Rozzini, R.; Barbisoni, P.; Morandi, A.; Ricci, A.; Giordano, A.; Trabucchi, M. Functional Trajectories during Hospitalization: A Prognostic Sign for Elderly Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Del Signore, S.; Fumagalli, S.; Gareri, P.; Malara, A.; Mossello, E.; Volpato, S.; Monzani, F.; Coin, A.; Bellelli, G.; et al. Assessing the Impact of COVID-19 on the Health of Geriatric Patients: The European GeroCovid Observational Study. Eur. J. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.M.; Ricks, M.; Fried, L.P.; Guralnik, J.M.; Xue, Q.-L.; Xia, J.; Bandeen-Roche, K. Functional Decline and Recovery of Activities of Daily Living in Hospitalized, Disabled Older Women: The Women’s Health and Aging Study, I. J. Am. Geriatr. Soc. 2009, 57, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- R&D Blueprint and COVID-19. Available online: https://www.who.int/teams/blueprint/covid-19 (accessed on 9 April 2021).
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A Standard Procedure for Creating a Frailty Index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Parmelee, P.A.; Thuras, P.D.; Katz, I.R.; Lawton, M.P. Validation of the Cumulative Illness Rating Scale in a Geriatric Residential Population. J. Am. Geriatr. Soc. 1995, 43, 130–137. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Carrillo-Garcia, P.; Garmendia-Prieto, B.; Cristofori, G.; Montoya, I.L.; Hidalgo, J.J.; Feijoo, M.Q.; Cortés, J.J.B.; Gómez-Pavón, J. Health Status in Survivors Older than 70 Years after Hospitalization with COVID-19: Observational Follow-up Study at 3 Months. Eur. Geriatr. Med. 2021, 12, 1091–1094. [Google Scholar] [CrossRef]
- Jovanoski, N.; Chen, X.; Becker, U.; Zalocusky, K.; Chawla, D.; Tsai, L.; Borm, M.; Neighbors, M.; Yau, V. Severity of COVID-19 and Adverse Long-Term Outcomes: A Retrospective Cohort Study Based on a US Electronic Health Record Database. BMJ Open 2021, 11, e056284. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.G.; Bruce, S.L.; Slater, C.L.; Tiao, J.R.; Baldwin, M.R.; Barr, R.G.; Chang, B.P.; Chau, K.H.; Choi, J.J.; Gavin, N.; et al. Characterization and Clinical Course of 1000 Patients with Coronavirus Disease 2019 in New York: Retrospective Case Series. BMJ 2020, 369, m1996. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Luppi, F.; Rebora, P.; D’Andrea, G.; Stainer, A.; Busnelli, S.; Catalano, M.; Modafferi, G.; Franco, G.; Monzani, A.; et al. One-Year Pulmonary Impairment after Severe COVID-19: A Prospective, Multicenter Follow-up Study. Respir. Res. 2022, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.K.; Joshi, D.; McMillan, J.; Erbas Oz, U.; Griffith, L.E.; Basta, N.E.; Kirkland, S.; Wolfson, C.; Raina, P. Assessment of Functional Mobility After COVID-19 in Adults Aged 50 Years or Older in the Canadian Longitudinal Study on Aging. JAMA Netw. Open 2022, 5, e2146168. [Google Scholar] [CrossRef]
- Ferrante, L.E.; Pisani, M.A.; Murphy, T.E.; Gahbauer, E.A.; Leo-Summers, L.S.; Gill, T.M. Functional Trajectories Among Older Persons Before and After Critical Illness. JAMA Intern. Med. 2015, 175, 523–529. [Google Scholar] [CrossRef]
- Andrew, M.K.; MacDonald, S.; Godin, J.; McElhaney, J.E.; LeBlanc, J.; Hatchette, T.F.; Bowie, W.; Katz, K.; McGeer, A.; Semret, M.; et al. Persistent Functional Decline Following Hospitalization with Influenza or Acute Respiratory Illness. J. Am. Geriatr. Soc. 2021, 69, 696–703. [Google Scholar] [CrossRef]


| Whole Cohort (n = 193) | Alive (n = 150) | Dead (n = 43) | p-Value | |
|---|---|---|---|---|
| Gender F (%) | 84 (43.5) | 58 (69.0) | 26 (31) | 0.011 |
| Age mean (SD) | 79.9 (9.1) | 78.1 (8.7) | 86.2 (7.7) | <0.001 |
| ADL median (IQR) | 6 (4) | 6 (0.25) | 2 (4) | <0.001 |
| Frailty status Robust (%) Prefrail (%) Frail (%) | 59 (30.6) 57 (29.5) 77 (39.9) | 52 (34.7) 44 (29.3) 54 (36.0) | 7 (16.3) 13 (30.2) 23 (53.5) | 0.044 |
| WHO status Mild disease (%) Moderate disease (%) Severe disease (%) Critical disease (%) | 41 (21.2) 103 (53.4) 42(21.8) 7 (3.6) | 35 (23.3) 79 (52.7) 29 (19.3) 7 (4.7) | 6 (14.0) 24 (55.8) 13 (30.2) 0 | 0.15 |
| NLR ratio baseline mean (SD) | 8.8 (17.3) | 9.2 (19.3) | 7.6 (5.6) | 0.61 |
| Hs-CRP mean (n.v. < 5 mg/L) | 77.3 (79.9) | 75.1 (79.1) | 82.2 (83.6) | 0.67 |
| Length of stay, median (days) | 15 (15) | 15 (11) | 15.5 (19) | 0.68 |
| Number of comorbidities, median (IQR) | 1 (2) | 1 (3) | 1 (2) | 0.32 |
| Arterial Hypertension (%) | 129 (67.2) | 101 (67.8) | 28 (65.1) | 0.74 |
| Atrial fibrillation (%) | 35 (18.2) | 28 (18.8) | 7 (16.3) | 0.70 |
| Heart failure (%) | 34 (17.7) | 28 (18.8) | 6(13.9) | 0.46 |
| Diabetes type 1 or type 2 (%) | 44 (22.9) | 31 (20.8) | 13(30.2) | 0.19 |
| Depression (%) | 24 (12.4) | 18 (12.0) | 6 (14.0) | 0.73 |
| COPD (%) | 32 (16.6) | 26 (17.3) | 6 (14.0) | 0.81 |
| Chronic Renal Failure (%) | 32 (16.6) | 25 (16.3) | 7 (16.6) | 0.95 |
| Chronic Liver Failure (%) | 5 (2.6) | 5 (100) | 0 | 0.22 |
| Obesity (%) | 16 (8.3) | 12 (8) | 4 (9.3) | 0.78 |
| Whole Cohort (n = 132) | Without ADL Lost (n = 104) | With ADL Lost (n = 28) | p-Value | |
|---|---|---|---|---|
| Gender F (%) | 46 (34.8) | 32 (69.6) | 14 (30.4) | 0.06 |
| Age mean (SD) | 77.4 (8.3) | 76.2 (7.9) | 81.8 (8.0) | 0.001 |
| ADL median (IQR) | 6 (1) | 6 (1) | 6 (1.5) | 0.56 |
| Frailty status Robust (%) Prefrail (%) Frail (%) | 49 (37.1) 40 (30.3) 43 (32.6) | 48 (98) 32 (80) 24 (55.8) | 1 (2) 8 (20) 19 (44.2) | <0.001 |
| WHO status at admission Mild disease (%) Moderate disease (%) Severe disease (%) Critical disease (%) | 32 (24.2) 86 (65.2) 4 (3.0) 10 (7.6) | 27 (84.4) 64(74.4) 3 (75.0) 10 (100) | 5(15.6) 22 (25.6) 1(25.0) 0(0) | 0.23 |
| P/F baseline mean (SD) | 266 (104) | 269 (109) | 255 (84) | 0.54 |
| NLR (SD) | 10.9 (22) | 10.6 (22) | 11.6 (21) | 0.84 |
| Hs-PCR (n.v. < 5 mg/L) | 76 (77.1) | 78.1 (84.2) | 68.7 (49.1) | 0.58 |
| Length of stay, median (days) | 14 (15) | 14 (11) | 20 (22) | 0.038 |
| Median Number of comorbidities (IQR) | 1 (3) | 1 (2) | 2 (2) | 0.036 |
| Arterial Hypertension (%) | 85 (64.4) | 64 (61.5) | 21 (75) | 0.37 |
| Atrial fibrillation (%) | 25 (18.9) | 19 (18.3) | 6 (21.4) | 0.78 |
| Cardiac failure (%) | 23 (17.4) | 16(15.4) | 7 (25) | 0.23 |
| Stroke (%) | 12 (8.1) | 11 (10.6) | 1 (3.6) | 0.46 |
| Diabetes type 1 or type 2 (%) | 28 (21.2) | 20 (19.2) | 8 (28.6) | 0.28 |
| Depression (%) | 15 (11.4) | 8 (7.7) | 7 (25) | 0.010 |
| COPD (%) | 12 (9.1) | 9 (8.7) | 3 (10.7) | 0.73 |
| Chronic Renal Failure (%) | 16 (16.9) | 11 (10.6) | 5 (17.9) | 0.29 |
| Chronic Liver Disease (%) | 1 (0.8) | 1(100) | 0 (0) | 0.60 |
| Obesity (%) | 8 (6.1) | 6 (5.78) | 2 (7.1) | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoye, C.; Calsolaro, V.; Calabrese, A.M.; Zotti, S.; Fedecostante, M.; Volpato, S.; Fumagalli, S.; Cherubini, A.; Antonelli Incalzi, R.; Monzani, F., on behalf of the GeroCovid Working Group. Determinants of Cause-Specific Mortality and Loss of Independence in Older Patients following Hospitalization for COVID-19: The GeroCovid Outcomes Study. J. Clin. Med. 2022, 11, 5578. https://doi.org/10.3390/jcm11195578
Okoye C, Calsolaro V, Calabrese AM, Zotti S, Fedecostante M, Volpato S, Fumagalli S, Cherubini A, Antonelli Incalzi R, Monzani F on behalf of the GeroCovid Working Group. Determinants of Cause-Specific Mortality and Loss of Independence in Older Patients following Hospitalization for COVID-19: The GeroCovid Outcomes Study. Journal of Clinical Medicine. 2022; 11(19):5578. https://doi.org/10.3390/jcm11195578
Chicago/Turabian StyleOkoye, Chukwuma, Valeria Calsolaro, Alessia Maria Calabrese, Sonia Zotti, Massimiliano Fedecostante, Stefano Volpato, Stefano Fumagalli, Antonio Cherubini, Raffaele Antonelli Incalzi, and Fabio Monzani on behalf of the GeroCovid Working Group. 2022. "Determinants of Cause-Specific Mortality and Loss of Independence in Older Patients following Hospitalization for COVID-19: The GeroCovid Outcomes Study" Journal of Clinical Medicine 11, no. 19: 5578. https://doi.org/10.3390/jcm11195578





