Edge-to-Edge Repair for Tricuspid Valve Regurgitation. Preliminary Echo-Data and Clinical Implications from the Tricuspid Regurgitation IMAging (TRIMA) Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
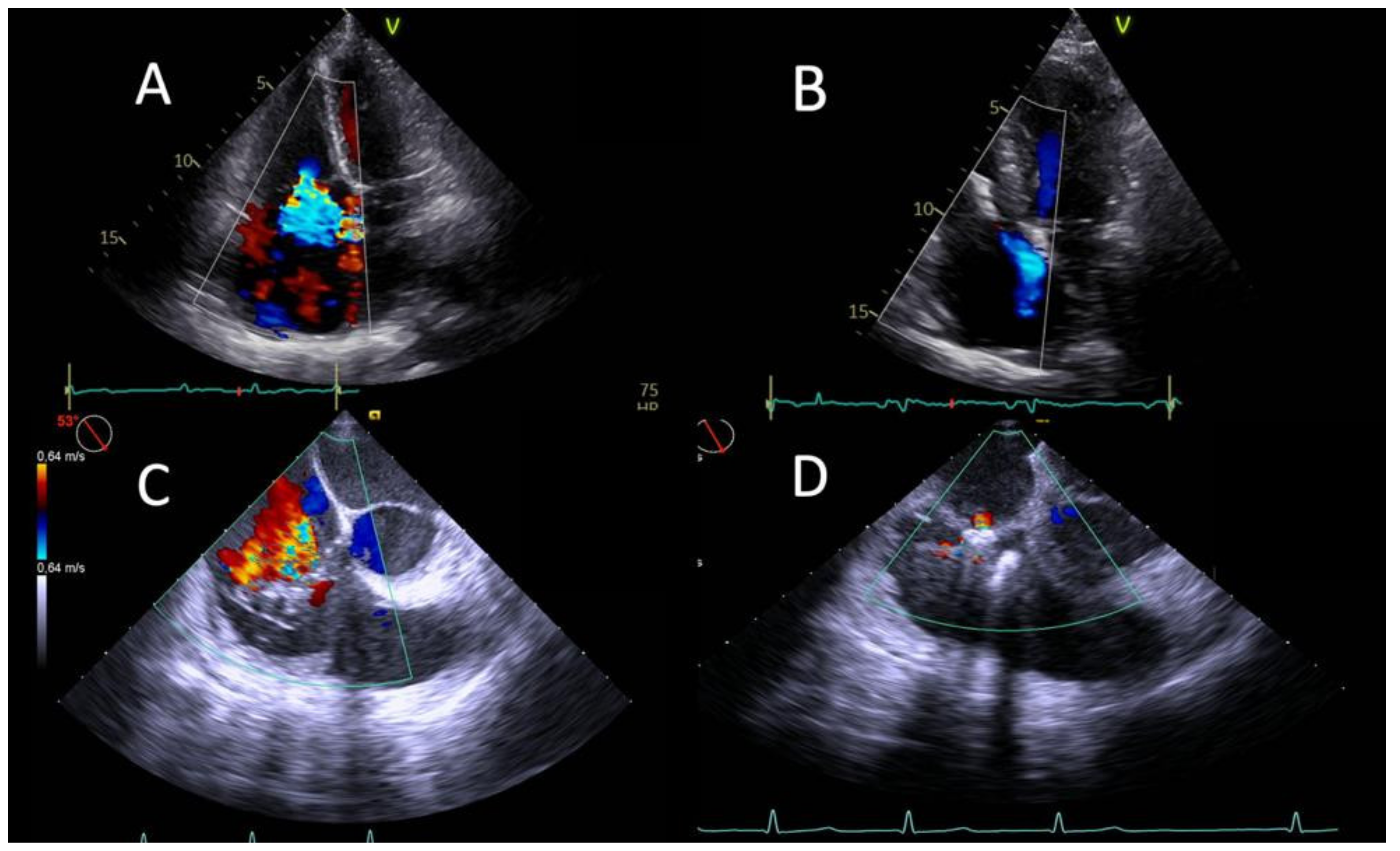
2.2. Echocardiography
2.3. Procedure
2.4. Outcomes and Endpoints
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural and Echocardiographic Results
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topilsky, Y.; Maltais, S.; Medina-Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.F.; Goedemans, L.; Vo, N.M.; Prihadi, E.A.; Van Der Bijl, P.; Gersh, B.J.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Significant Isolated Tricuspid Regurgitation in Patients with Atrial Fibrillation without Left-Sided Heart Disease or Pulmonary Hypertension. Am. J. Cardiol. 2020, 135, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Lee, J.-W.; Song, J.-M.; Park, J.P.; Lee, J.W.; Kang, D.-H.; Song, J.-K. Long-Term Prognosis of Isolated Significant Tricuspid Regurgitation. Circ. J. 2010, 74, 375–380. [Google Scholar] [CrossRef]
- Dreyfus, J.; Flagiello, M.; Bazire, B.; Eggenspieler, F.; Viau, F.; Riant, E.; Mbaki, Y.; Bohbot, Y.; Eyharts, D.; Senage, T.; et al. Isolated tricuspid valve surgery: Impact of aetiology and clinical presentation on outcomes. Eur. Heart J. 2020, 41, 4304–4317. [Google Scholar] [CrossRef]
- Axtell, A.L.; Bhambhani, V.; Moonsamy, P.; Healy, E.W.; Picard, M.H.; Sundt, T.M.; Wasfy, J.H. Surgery Does Not Improve Survival in Patients with Isolated Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 715–725. [Google Scholar] [CrossRef]
- Curio, J.; Demir, O.M.; Pagnesi, M.; Mangieri, A.; Giannini, F.; Weisz, G.; Latib, A. Update on the Current Landscape of Transcatheter Options for Tricuspid Regurgitation Treatment. Interv. Cardiol. Rev. Res. Resour. 2019, 14, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mehr, M.; Taramasso, M.; Besler, C.; Ruf, T.; Connelly, K.A.; Weber, M.; Yzeiraj, E.; Schiavi, D.; Mangieri, A.; Vaskelyte, L.; et al. 1-Year Outcomes After Edge-to-Edge Valve Repair for Symptomatic Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2019, 12, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; von Bardeleben, R.S.; Weber, M.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.-N.; Nabauer, M.; Tang, G.H.; et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Curio, J.; Lanzillo, G.; Mangieri, A.; Pagnesi, M.; Montalto, C.; Demir, O.M.; De Bonis, M.; Agricola, E.; Colombo, A.; Latib, A. Transcatheter Interventions for Severe TR Patients Presenting to a Tertiary Care Setting. J. Am. Coll. Cardiol. 2019, 74, 821–823. [Google Scholar] [CrossRef]
- Cammalleri, V.; Carpenito, M.; Bono, M.C.; Mega, S.; Ussia, G.P.; Grigioni, F. Transcatheter Tricuspid Valve Therapy: From Anatomy to Intervention. Front. Cardiovasc. Med. 2021, 8, 778445. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Mega, S.; Ussia, G.P.; Grigioni, F. Mitral and Tricuspid Valves Percutaneous Repair in Patients with Advanced Heart Failure. Heart Fail. Clin. 2021, 17, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Carpenito, M.; De Stefano, D.; Ussia, G.P.; Bono, M.C.; Mega, S.; Nusca, A.; Cocco, N.; Nobile, E.; De Filippis, A.; et al. Novel Computed Tomography Variables for Assessing Tricuspid Valve Morphology: Results from the TRIMA (Tricuspid Regurgitation IMAging) Study. J. Clin. Med. 2022, 11, 2825. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Hahn, R.T.; Zamorano, J.L. The need for a new tricuspid regurgitation grading scheme. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1342–1343. [Google Scholar] [CrossRef]
- Hahn, R.T.; Badano, L.P.; Bartko, P.E.; Muraru, D.; Maisano, F.; Zamorano, J.L.; Donal, E. Tricuspid regurgitation: Recent advances in understanding pathophysiology, severity grading and outcome. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 913–929. [Google Scholar] [CrossRef]
- Ohno, Y.; Attizzani, G.F.; Capodanno, D.; Cannata, S.; Dipasqua, F.; Immé, S.; Barbanti, M.; Ministeri, M.; Caggegi, A.; Pistritto, A.M.; et al. Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip System: 30-day and 12-month follow-up from the GRASP Registry. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1246–1255. [Google Scholar] [CrossRef]
- Lindman, B.R.; Maniar, H.S.; Jaber, W.A.; Lerakis, S.; Mack, M.J.; Suri, R.M.; Thourani, V.H.; Babaliaros, V.; Kereiakes, D.J.; Whisenant, B.; et al. Effect of Tricuspid Regurgitation and the Right Heart on Survival After Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2015, 8, e002073. [Google Scholar] [CrossRef]
- Besler, C.; Orban, M.; Rommel, K.-P.; Braun, D.; Patel, M.; Hagl, C.; Borger, M.; Nabauer, M.; Massberg, S.; Thiele, H.; et al. Predictors of Procedural and Clinical Outcomes in Patients with Symptomatic Tricuspid Regurgitation Undergoing Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2018, 11, 1119–1128. [Google Scholar] [CrossRef]
- Orban, M.; Wolff, S.; Braun, D.; Stolz, L.; Higuchi, S.; Stark, K.; Mehr, M.; Stocker, T.J.; Dischl, D.; Scherer, C.; et al. Right Ventricular Function in Transcatheter Edge-to-Edge Tricuspid Valve Repair. JACC Cardiovasc. Imaging 2021, 14, 2477–2479. [Google Scholar] [CrossRef]
- Kresoja, K.-P.; Rommel, K.-P.; Lücke, C.; Unterhuber, M.; Besler, C.; von Roeder, M.; Schöber, A.R.; Noack, T.; Gutberlet, M.; Thiele, H.; et al. Right Ventricular Contraction Patterns in Patients Undergoing Transcatheter Tricuspid Valve Repair for Severe Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2021, 14, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Weber, M.; Lurz, P.; von Bardeleben, R.S.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.-N.; Näbauer, M.; et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2019, 394, 2002–2011. [Google Scholar] [CrossRef]
- Taramasso, M.; Benfari, G.; van der Bijl, P.; Alessandrini, H.; Attinger-Toller, A.; Biasco, L.; Lurz, P.; Braun, D.; Brochet, E.; Connelly, K.A.; et al. Transcatheter Versus Medical Treatment of Patients with Symptomatic Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2998–3008. [Google Scholar] [CrossRef]
- Sugiura, A.; Tanaka, T.; Kavsur, R.; Oeztuerk, C.; Vogelhuber, J.; Wilde, N.; Becher, M.U.; Zimmer, S.; Nickenig, G.; Weber, M. Leaflet Configuration and Residual Tricuspid Regurgitation After Transcatheter Edge-to-Edge Tricuspid Repair. JACC Cardiovasc. Interv. 2021, 14, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Rommel, K.-P.; Besler, C.; Noack, T.; Blazek, S.; von Roeder, M.; Fengler, K.; Ender, J.; Gutberlet, M.; Desch, S.; Borger, M.A.; et al. Physiological and Clinical Consequences of Right Ventricular Volume Overload Reduction After Transcatheter Treatment for Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2019, 12, 1423–1434. [Google Scholar] [CrossRef]
- Fortuni, F.; Butcher, S.C.; Dietz, M.F.; van der Bijl, P.; Prihadi, E.A.; De Ferrari, G.M.; Marsan, N.A.; Bax, J.J.; Delgado, V. Right Ventricular–Pulmonary Arterial Coupling in Secondary Tricuspid Regurgitation. Am. J. Cardiol. 2021, 148, 138–145. [Google Scholar] [CrossRef]
- Sugiura, A.; Kavsur, R.; Spieker, M.; Iliadis, C.; Mauri, V.; Tanaka, T.; Goto, T.; Weber, M.; Kelm, M.; Baldus, S.; et al. Impact of right ventricular-pulmonary arterial coupling on clinical outcomes of tricuspid regurgitation. EuroIntervention 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Brener, M.I.; Lurz, P.; Hausleiter, J.; Rodés-Cabau, J.; Fam, N.; Kodali, S.K.; Rommel, K.-P.; Muntané-Carol, G.; Gavazzoni, M.; Nazif, T.M.; et al. Right Ventricular-Pulmonary Arterial Coupling and Afterload Reserve in Patients Undergoing Transcatheter Tricuspid Valve Repair. J. Am. Coll. Cardiol. 2022, 79, 448–461. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure with Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | N = 13 |
|---|---|
| Mean age, years ± SD | 81 ± 4 |
| Gender-female, n (%) | 11 (85) |
| BSA, m2 ± SD | 1.7 ± 0.2 |
| STS score, % median (Q1–Q3) | 5.8 (3.8–7.7) |
| EuroScore II, % ± SD | 8 ± 4 |
| Diabetes, n (%) | 4 (31) |
| Hypertension, n (%) | 12 (92) |
| Hyperlipidaemia, n (%) | 5 (38) |
| Coronary artery disease, n (%) | 4 (31) |
| Smoking, n (%) | 1 (8) |
| Atrial fibrillation, n (%) | 13 (100) |
| History of cerebrovascular insult, n (%) | 2 (15) |
| COPD, n (%) | 2 (15) |
| CIED, n (%) | 4 (31) |
| Previous TAVI implantation, n (%) | 3 (23) |
| NYHA functional class III or more, n (%) | 13 (100) |
| Medications | |
| Salicylic Acid, n (%) | 3 (23) |
| Novel oral anticoagulant, n (%) | 8 (62) |
| Warfarin, n (%) | 4 (31) |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, n (%) | 6 (46) |
| Digoxin, n (%) | 3 (23) |
| Calcium channel blocker, n (%) | 3 (23) |
| Beta-blocker, n (%) | 11 (85) |
| Diuretic, n (%) | 13 (100) |
| Echocardiographic Parameter | Pre-TriClip | Post-TriClip | p Value |
|---|---|---|---|
| LVEF, % ± SD | 50 ± 7 | 49 ± 8 | 0.473 |
| TAPSE, mm ± SD | 16 ± 3 | 17 ± 3 | 0.150 |
| S’ wave, cm/s ± SD | 9.3 ± 2.4 | 9.5 ± 1.5 | 0.732 |
| FAC, % ± SD | 33 ± 8 | 36 ± 8 | 0.370 |
| PASP, mmHg ± SD | 43 ± 9.5 | 36.5 ± 9.1 | 0.034 |
| TAPSE/PASP, mm/mmHg ± SD | 0.37 ± 0.1 | 0.46 ± 0.1 | 0.011 |
| TR grade | |||
| 1 | 46% (6) | 0.001 | |
| 2 | 46% (6) | ||
| 3 | 69% (9) | ||
| 4 | 23% (3) | 8% (1) | |
| 5 | 8% (1) | ||
| TR VC, mm ± SD | 8 ± 1 | 4 ± 2 | <0.001 |
| TR EROA, cm2 ± SD | 0.63 ± 0.28 | 0.32 ± 0.21 | <0.001 |
| TV mean diastolic gradient, mmHg ± SD | 0.9 ± 0.6 | 1.9 ± 1.1 | 0.004 |
| TV V max, m/s ± SD | 2.8 ± 0.7 | 2.5 ± 0.5 | 0.105 |
| TR volume, mL ± SD | 57 ± 16 | 28 ± 16 | <0.001 |
| Tricuspid annulus diameter, mm ± SD | 44 ± 5 | 40 ± 4 | <0.001 |
| RV length, mm ± SD | 60 ± 7 | 56 ± 6 | 0.08 |
| RV middle diameter, mm ± SD | 42 ± 6 | 35 ± 6 | 0.003 |
| RV basal diameter, mm ± SD | 47 ± 7 | 43 ± 4 | 0.001 |
| RV end diastolic area, cm2 ± SD | 19 ± 4 | 16 ± 3 | 0.049 |
| RA area, cm2 ± SD | 28 ± 8 | 26 ± 8 | 0.026 |
| IVC, mm ± SD | 22 ± 3 | 18 ± 5 | 0.004 |
| Adverse Events and Clinical Status (N, %) | 30-Day Follow-Up (N = 13) | 6-Month Follow-Up (N = 10) |
|---|---|---|
| Cardiovascular mortality | 0 | 0 |
| Myocardial infarction | 0 | 0 |
| Stroke | 0 | 0 |
| New onset renal failure | 0 | 0 |
| Nonelective surgery for tricuspid valve repair | 0 | 0 |
| Endocarditis requiring surgery | 0 | 0 |
| Major bleeding 1 | 0 | 0 |
| New onset of liver failure | 0 | 0 |
| Pulmonary thromboembolism | 0 | 0 |
| Device embolisation | 0 | 0 |
| Single leaflet device attachment | 1 (13%) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpenito, M.; Cammalleri, V.; Vitez, L.; De Filippis, A.; Nobile, E.; Bono, M.C.; Mega, S.; Bunc, M.; Grigioni, F.; Ussia, G.P. Edge-to-Edge Repair for Tricuspid Valve Regurgitation. Preliminary Echo-Data and Clinical Implications from the Tricuspid Regurgitation IMAging (TRIMA) Study. J. Clin. Med. 2022, 11, 5609. https://doi.org/10.3390/jcm11195609
Carpenito M, Cammalleri V, Vitez L, De Filippis A, Nobile E, Bono MC, Mega S, Bunc M, Grigioni F, Ussia GP. Edge-to-Edge Repair for Tricuspid Valve Regurgitation. Preliminary Echo-Data and Clinical Implications from the Tricuspid Regurgitation IMAging (TRIMA) Study. Journal of Clinical Medicine. 2022; 11(19):5609. https://doi.org/10.3390/jcm11195609
Chicago/Turabian StyleCarpenito, Myriam, Valeria Cammalleri, Luka Vitez, Aurelio De Filippis, Edoardo Nobile, Maria Caterina Bono, Simona Mega, Matjaz Bunc, Francesco Grigioni, and Gian Paolo Ussia. 2022. "Edge-to-Edge Repair for Tricuspid Valve Regurgitation. Preliminary Echo-Data and Clinical Implications from the Tricuspid Regurgitation IMAging (TRIMA) Study" Journal of Clinical Medicine 11, no. 19: 5609. https://doi.org/10.3390/jcm11195609





