The Importance of the Assessment of Epicardial Adipose Tissue in Scientific Research
Abstract
1. Introduction
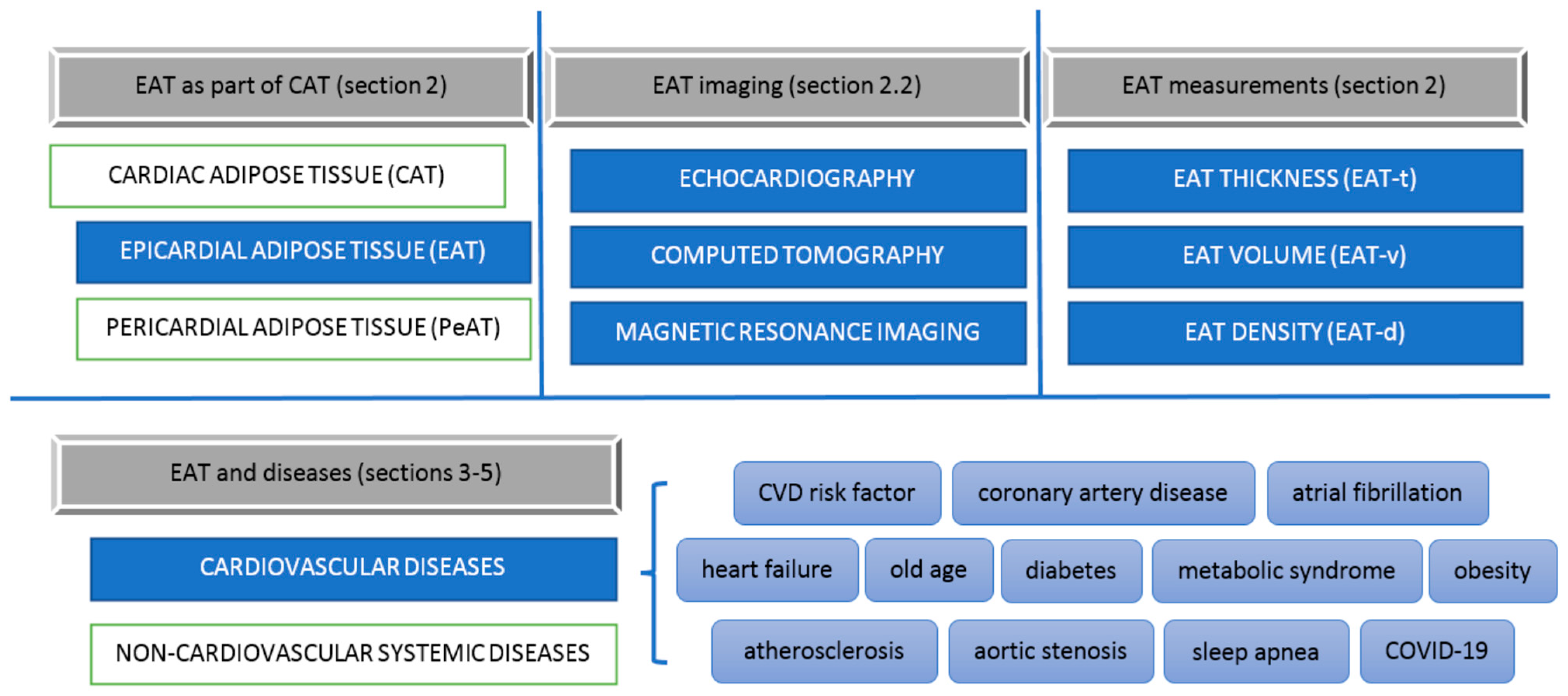
2. Cardiac Adipose Tissue
2.1. Distribution of the Epicardial Adipose Tissue
2.2. Imaging and Measurement Methods of Epicardial Adipose Tissue
3. Epicardial Adipose Tissue in CLINICAL Medicine
3.1. Epicardial Adipose Tissue and Atrial Fibrillation Recurrence
3.2. Epicardial Adipose Tissue and Relation to Metabolism in Old Patients
4. Clinical Practice and Studies Focused on Heart Adipose
5. Epicardial Adipose Tissue and COVID-19-Related Cardiac Syndrome
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toczyłowski, K.; Gruca, M.; Baranowski, M. Epicardial Adipose Tissue and Its Role in Cardiac Physiology and Disease. Postępy Hig. Med. Dośw. 2013, 67, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Murawska-Ciałowicz, E. Adipose Tissue–Morphological and Biochemical Characteristic of Different Depots. Postępy Hig. Med. Dośw. 2017, 71, 466–484. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The Adipose Organ. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.; Grzelak, T.; Czyżewska, K. Endocrine function of adipose tissue in historical perspective. Hygeia Public Health 2016, 51, 339–342. [Google Scholar]
- de Feyter, P.J. Epicardial Adipose Tissue: An Emerging Role for the Development of Coronary Atherosclerosis. Clin. Cardiol. 2011, 34, 143–144. [Google Scholar] [CrossRef]
- Gaborit, B.; Kober, F.; Jacquier, A.; Moro, P.J.; Cuisset, T.; Boullu, S.; Dadoun, F.; Alessi, M.-C.; Morange, P.; Clément, K.; et al. Assessment of Epicardial Fat Volume and Myocardial Triglyceride Content in Severely Obese Subjects: Relationship to Metabolic Profile, Cardiac Function and Visceral Fat. Int. J. Obes. 2012, 36, 422–430. [Google Scholar] [CrossRef]
- Iozzo, P. Myocardial, Perivascular, and Epicardial Fat. Diabetes Care 2011, 34, S371–S379. [Google Scholar] [CrossRef]
- Kłoda, K.; Mierzecki, A. The Less Adipose Tissue the Better? Lek. POZ 2021, 7, 352–387. Available online: https://www.termedia.pl/Im-mniej-tkanki-tluszczowej-tym-lepiej-,98,45712,0,0.html (accessed on 21 August 2022).
- Stupnicki, R.; Tomaszewski, P. Body mass index and body fat content in adults. Hygeia Public Health 2016, 51, 335–338. [Google Scholar]
- Filipiak, J.K. The commentary. The epicardial adipose tissue—what we know about the role of statins in reducing it? Chor. Serca Naczyń 2015, 12, 205–206. [Google Scholar]
- Reiner, L.; Mazzoleni, A.; Rodriguez, F.L. Statistical Analysis of the Epicardial Fat Weight in Human Hearts. AMA Arch. Pathol. 1955, 60, 369–373. [Google Scholar] [PubMed]
- Zhou, H.; An, D.-A.; Ni, Z.; Xu, J.; Zhou, Y.; Fang, W.; Lu, R.; Ying, L.; Huang, J.; Yao, Q.; et al. Magnetic Resonance Imaging Quantification of Accumulation of Epicardial Adipose Tissue Adds Independent Risks for Diastolic Dysfunction among Dialysis Patients. J. Magn. Reson. Imaging 2022, 56, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Flüchter, S.; Haghi, D.; Dinter, D.; Heberlein, W.; Kühl, H.P.; Neff, W.; Sueselbeck, T.; Borggrefe, M.; Papavassiliu, T. Volumetric Assessment of Epicardial Adipose Tissue with Cardiovascular Magnetic Resonance Imaging. Obesity 2007, 15, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Barbaro, G. Epicardial Adipose Tissue Feeding and Overfeeding the Heart. Nutrition 2019, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial Adipose Tissue as a Metabolic Transducer: Role in Heart Failure and Coronary Artery Disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef]
- Shmilovich, H.; Dey, D.; Cheng, V.Y.; Rajani, R.; Nakazato, R.; Otaki, Y.; Nakanishi, R.; Slomka, P.J.; Thomson, L.E.J.; Hayes, S.W.; et al. Threshold for the Upper Normal Limit of Indexed Epicardial Fat Volume: Derivation in a Healthy Population and Validation in an Outcome-Based Study. Am. J. Cardiol. 2011, 108, 1680–1685. [Google Scholar] [CrossRef]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends in endocrinology and metabolism. Trends Endocrinol Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef]
- Ngo, D.T.; Gokce, N. Epicardial Adipose Tissue: A Benign Consequence of Obesity? Circ. Cardiovasc. Imaging 2015, 8, e003156. [Google Scholar] [CrossRef]
- Eroğlu, S. How Do We Measure Epicardial Adipose Tissue Thickness by Transthoracic Echocardiography? Anatol. J. Cardiol. 2015, 15, 416–419. [Google Scholar] [CrossRef]
- Sova, M.; Genzor, S.; Kolek, V.; Čtvrtlík, F.; Asswad, A.G.; Zela, O.; Tauber, Z. Epicardial Fat in Patients with Chronic Obstructive Pulmonary Disease as a Marker of High Cardiovascular Risk-Review. Adv. Respir. Med. 2018, 86, 314–318. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Lehmann, N.; Kälsch, H.; Bauer, M.; Dykun, I.; Kara, K.; Moebus, S.; Jöckel, K.-H.; Erbel, R.; Möhlenkamp, S. Association of Epicardial Adipose Tissue and Left Atrial Size on Non-Contrast CT with Atrial Fibrillation: The Heinz Nixdorf Recall Study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, O.; Kurtoglu, E.; Gozubuyuk, G.; Dogan, C.; Acar, Z.; EyupKoca, F.; Pekdemir, H. Epicardial Adipose Tissue Thickness in Patients with Chronic Obstructive Pulmonary Disease Having Right Ventricular Systolic Dysfunction. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2461–2467. [Google Scholar] [PubMed]
- Mahajan, R.; Kuklik, P.; Grover, S.; Brooks, A.G.; Wong, C.X.; Sanders, P.; Selvanayagam, J.B. Cardiovascular Magnetic Resonance of Total and Atrial Pericardial Adipose Tissue: A Validation Study and Development of a 3 Dimensional Pericardial Adipose Tissue Model. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2013, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Ganesan, A.N.; Selvanayagam, J.B. Epicardial Fat and Atrial Fibrillation: Current Evidence, Potential Mechanisms, Clinical Implications, and Future Directions. Eur. Heart J. 2017, 38, 1294–1302. [Google Scholar] [CrossRef]
- Saremi, F.; Mekhail, S.; Sefidbakht, S.; Thonar, B.; Malik, S.; Sarlaty, T. Quantification of Epicardial Adipose Tissue: Correlation of Surface Area and Volume Measurements. Acad. Radiol. 2011, 18, 977–983. [Google Scholar] [CrossRef]
- Park, M.J.; Jung, J.I.; Oh, Y.S.; Youn, H.-J. Assessment of Epicardial Fat Volume with Threshold-Based 3-Dimensional Segmentation in CT: Comparison with the 2-Dimensional Short Axis-Based Method. Korean Circ. J. 2010, 40, 328–333. [Google Scholar] [CrossRef]
- Szymański, F.M. Epicardial adipose tissue in the pathogenesis of the cardiovascular disease-should we consider it as a cardiovascular risk factor and strive to reduce its amount? Chor. Serca Naczyń 2015, 12, 199–204. [Google Scholar]
- Chruściel, P.; Banach, M. Atorvastatin in patients with overweight and obesity. Chor. Serca Naczyń 2016, 13, 5–14. [Google Scholar]
- Ito, T.; Nasu, K.; Terashima, M.; Ehara, M.; Kinoshita, Y.; Ito, T.; Kimura, M.; Tanaka, N.; Habara, M.; Tsuchikane, E.; et al. The Impact of Epicardial Fat Volume on Coronary Plaque Vulnerability: Insight from Optical Coherence Tomography Analysis. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 408–415. [Google Scholar] [CrossRef]
- Yerramasu, A.; Dey, D.; Venuraju, S.; Anand, D.V.; Atwal, S.; Corder, R.; Berman, D.S.; Lahiri, A. Increased Volume of Epicardial Fat Is an Independent Risk Factor for Accelerated Progression of Sub-Clinical Coronary Atherosclerosis. Atherosclerosis 2012, 220, 223–230. [Google Scholar] [CrossRef]
- Picard, F.A.; Gueret, P.; Laissy, J.-P.; Champagne, S.; Leclercq, F.; Carrié, D.; Juliard, J.-M.; Henry, P.; Niarra, R.; Chatellier, G.; et al. Epicardial Adipose Tissue Thickness Correlates with the Presence and Severity of Angiographic Coronary Artery Disease in Stable Patients with Chest Pain. PLoS ONE 2014, 9, e110005. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Fukuda, S.; Tanaka, A.; Otsuka, K.; Jissho, S.; Taguchi, H.; Yoshikawa, J.; Shimada, K. Persistent Epicardial Adipose Tissue Accumulation Is Associated with Coronary Plaque Vulnerability and Future Acute Coronary Syndrome in Non-Obese Subjects with Coronary Artery Disease. Atherosclerosis 2014, 237, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kleinrok, A.; Głowa, B. The Obesity and Its Meaning in Cardiovascular Diseases Part 1. Obesity as a Risk Factor. Med. Rev. 2015, 13, 165–172. [Google Scholar] [CrossRef]
- Okada, K.; Ohshima, S.; Isobe, S.; Harada, K.; Hirashiki, A.; Funahashi, H.; Arai, K.; Hayashi, D.; Hayashi, M.; Ishii, H.; et al. Epicardial Fat Volume Correlates with Severity of Coronary Artery Disease in Nonobese Patients. J. Cardiovasc. Med. Hagerstown Md 2014, 15, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Demircelik, M.B.; Yilmaz, O.C.; Gurel, O.M.; Selcoki, Y.; Atar, I.A.; Bozkurt, A.; Akin, K.; Eryonucu, B. Epicardial Adipose Tissue and Pericoronary Fat Thickness Measured with 64-Multidetector Computed Tomography: Potential Predictors of the Severity of Coronary Artery Disease. Clinics 2014, 69, 388–392. [Google Scholar] [CrossRef]
- Yamashita, K.; Yamamoto, M.H.; Igawa, W.; Ono, M.; Kido, T.; Ebara, S.; Okabe, T.; Saito, S.; Amemiya, K.; Isomura, N.; et al. Association of Epicardial Adipose Tissue Volume and Total Coronary Plaque Burden in Patients with Coronary Artery Disease. Int. Heart J. 2018, 59, 1219–1226. [Google Scholar] [CrossRef]
- Mazurek, T. Nasierdziowa Tkanka Tłuszczowa a Przepływ w Tętnicach Wieńcowych. Czy Lokalizacja Ma Znaczenie? Kardiol. Pol. Pol. Heart J. 2012, 70, 910. [Google Scholar]
- Cheng, K.-H.; Chu, C.-S.; Lee, K.-T.; Lin, T.-H.; Hsieh, C.-C.; Chiu, C.-C.; Voon, W.-C.; Sheu, S.-H.; Lai, W.-T. Adipocytokines and Proinflammatory Mediators from Abdominal and Epicardial Adipose Tissue in Patients with Coronary Artery Disease. Int. J. Obes. 2008, 32, 268–274. [Google Scholar] [CrossRef]
- Alexopoulos, N.; McLean, D.S.; Janik, M.; Arepalli, C.D.; Stillman, A.E.; Raggi, P. Epicardial Adipose Tissue and Coronary Artery Plaque Characteristics. Atherosclerosis 2010, 210, 150–154. [Google Scholar] [CrossRef]
- Bertaso, A.G.; Bertol, D.; Duncan, B.B.; Foppa, M. Epicardial Fat: Definition, Measurements and Systematic Review of Main Outcomes. Arq. Bras. Cardiol. 2013, 101, e18–e28. [Google Scholar] [CrossRef]
- Uygur, B.; Celik, O.; Ozturk, D.; Erturk, M.; Otcu, H.; Ustabasıoglu, F.E.; Yıldırım, A. The Relationship between Location-Specific Epicardial Adipose Tissue Volume and Coronary Atherosclerotic Plaque Burden in Type 2 Diabetic Patients. Kardiol. Pol. 2017, 75, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Greif, M.; Becker, A.; von Ziegler, F.; Lebherz, C.; Lehrke, M.; Broedl, U.C.; Tittus, J.; Parhofer, K.; Becker, C.; Reiser, M.; et al. Pericardial Adipose Tissue Determined by Dual Source CT Is a Risk Factor for Coronary Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Janik, M.; Hartlage, G.; Alexopoulos, N.; Mirzoyev, Z.; McLean, D.S.; Arepalli, C.D.; Chen, Z.; Stillman, A.E.; Raggi, P. Epicardial Adipose Tissue Volume and Coronary Artery Calcium to Predict Myocardial Ischemia on Positron Emission Tomography-Computed Tomography Studies. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2010, 17, 841–847. [Google Scholar] [CrossRef]
- Kalaycıoğlu, E.; Çetin, M.; Çinier, G.; Özyıldız, A.G.; Durmuş, İ.; Kırış, T.; Gökdeniz, T. Epicardial Adipose Tissue Is Associated with Increased Systolic Pulmonary Artery Pressure in Patients with Chronic Obstructive Pulmonary Disease. Clin. Respir. J. 2021, 15, 406–412. [Google Scholar] [CrossRef]
- Zagaceta, J.; Zulueta, J.J.; Bastarrika, G.; Colina, I.; Alcaide, A.B.; Campo, A.; Celli, B.R.; de Torres, J.P. Epicardial Adipose Tissue in Patients with Chronic Obstructive Pulmonary Disease. PLoS ONE 2013, 8, e65593. [Google Scholar] [CrossRef]
- Demir, M.; Acet, H.; Kaya, H.; Taylan, M.; Yüksel, M.; Yılmaz, S.; Sezgi, C.; Karadeniz, G.; Yenibertiz, D. Relationship between Metabolic Syndrome and Epicardial Fat Tissue Thickness in Patients with Chronic Obstructive Pulmonary Disease. Anatol. J. Cardiol. 2016, 16, 405–411. [Google Scholar] [CrossRef]
- Kiraz, K.; Gökdeniz, T.; Kalaycıoglu, E.; Börekçi, A.; Akyol, S.; Baykan, A.O.; Acele, A.; Karakoyun, S.; Seker, T.; Gür, M. Epicardial Fat Thickness Is Associated with Severity of Disease in Patients with Chronic Obstructive Pulmonary Disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4508–4515. [Google Scholar]
- Ding, J.; Hsu, F.-C.; Harris, T.B.; Liu, Y.; Kritchevsky, S.B.; Szklo, M.; Ouyang, P.; Espeland, M.A.; Lohman, K.K.; Criqui, M.H.; et al. The Association of Pericardial Fat with Incident Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2009, 90, 499–504. [Google Scholar] [CrossRef]
- van den Borst, B.; Gosker, H.R.; Schols, A.M.W.J. Central Fat and Peripheral Muscle: Partners in Crime in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 8–13. [Google Scholar] [CrossRef]
- van den Borst, B.; Gosker, H.R.; Koster, A.; Yu, B.; Kritchevsky, S.B.; Liu, Y.; Meibohm, B.; Rice, T.B.; Shlipak, M.; Yende, S.; et al. The Influence of Abdominal Visceral Fat on Inflammatory Pathways and Mortality Risk in Obstructive Lung Disease. Am. J. Clin. Nutr. 2012, 96, 516–526. [Google Scholar] [CrossRef]
- Unubol, M.; Eryilmaz, U.; Guney, E.; Akgullu, C.; Kurt Omurlu, I. Epicardial Adipose Tissue in Patients with Subclinical Hypothyroidism. Minerva Endocrinol. 2014, 39, 135–140. [Google Scholar] [PubMed]
- Sayin, I.; Erkan, A.F.; Ekici, B.; Kutuk, U.; Corakci, A.; Tore, H.F. Thickening of the Epicardial Adipose Tissue Can Be Alleviated by Thyroid Hormone Replacement Therapy in Patients with Subclinical Hypothyroidism. Kardiol. Pol. 2016, 74, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, L.; Sahin, S.; Akyuz, A.R.; Ziyrek, M.; Anaforoglu, I.; Kose, M.; Erkan, H.; Ağaç, M.T.; Acar, Z. Epicardial Adipose Tissue Increased in Patients with Newly Diagnosed Subclinical Hypothyroidism. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2013, 22, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Asik, M.; Sahin, S.; Ozkul, F.; Anaforoglu, I.; Ayhan, S.; Karagol, S.; Gunes, F.; Algun, E. Evaluation of Epicardial Fat Tissue Thickness in Patients with Hashimoto Thyroiditis. Clin. Endocrinol. 2013, 79, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, D.; Özben, B.; Toprak, A.; Yavuz, D.; Aydın, H.; Tarçın, Ö.; Deyneli, O.; Akalın, S. Effects of Restoration of the Euthyroid State on Epicardial Adipose Tissue and Carotid Intima Media Thickness in Subclinical Hypothyroid Patients. Endocrine 2015, 48, 909–915. [Google Scholar] [CrossRef]
- Santos, O.C.; Silva, N.A.O.; Vaisman, M.; Turano, M.D.; Dytz, M.G.; Huber, G.A.; Braulio, V.B.; Teixeira, P.F.S. Evaluation of Epicardial Fat Tissue Thickness as a Marker of Cardiovascular Risk in Patients with Subclinical Hypothyroidism. J. Endocrinol. Investig. 2015, 38, 421–427. [Google Scholar] [CrossRef]
- Wójcik-Cichy, K.; Piekarska, A. Influence of non-alcoholic fatty liver disease on risk of atherosclerosis and cardiovascular diseases. Hepatologia 2017, 17, 13–17. [Google Scholar] [CrossRef]
- Pisto, P.; Santaniemi, M.; Bloigu, R.; Ukkola, O.; Kesäniemi, Y.A. Fatty Liver Predicts the Risk for Cardiovascular Events in Middle-Aged Population: A Population-Based Cohort Study. BMJ Open 2014, 4, e004973. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Li, Y.; Liu, Y.; Yan, Y.; Luo, A.; Ren, H.; She, Q. Association of Epicardial Adipose Tissue with Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Hepatol. Int. 2019, 13, 757–765. [Google Scholar] [CrossRef]
- Fargion, S.; Porzio, M.; Fracanzani, A.L. Nonalcoholic Fatty Liver Disease and Vascular Disease: State-of-the-Art. World J. Gastroenterol. 2014, 20, 13306–13324. [Google Scholar] [CrossRef]
- Chen, J.; Mei, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zeng, R.; Liu, Q. Epicardial Adipose Tissue Is Associated with Higher Recurrence Risk after Catheter Ablation in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2022, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, U.; Aytemir, K.; Yorgun, H.; Asil, S.; Dural, M.; Özer, N. The Impact of Echocardiographic Epicardial Fat Thickness on Outcomes of Cryoballoon-Based Atrial Fibrillation Ablation. Echocardiography 2016, 33, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-F.; Hung, C.-L.; Tsao, H.-M.; Lin, Y.-J.; Yun, C.-H.; Lai, Y.-H.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; et al. Epicardial Adipose Tissue Thickness and Ablation Outcome of Atrial Fibrillation. PLoS ONE 2013, 8, e74926. [Google Scholar] [CrossRef] [PubMed]
- Sanghai, S.R.; Sardana, M.; Hansra, B.; Lessard, D.M.; Dahlberg, S.T.; Aurigemma, G.P.; Fitzgibbons, T.P.; McManus, D.D. Indexed Left Atrial Adipose Tissue Area Is Associated with Severity of Atrial Fibrillation and Atrial Fibrillation Recurrence among Patients Undergoing Catheter Ablation. Front. Cardiovasc. Med. 2018, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Yamada, T.; Furukawa, Y.; Morita, T.; Tamaki, S.; Kida, H.; Sakata, Y.; Fukunami, M. Are Cardiac Sympathetic Nerve Activity and Epicardial Adipose Tissue Associated with Atrial Fibrillation Recurrence after Catheter Ablation in Patients without Heart Failure? Int. J. Cardiol. 2020, 303, 41–48. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Poggio, P.; Valerio, V.; Cabaro, S.; Campana, P.; Comentale, G.; Attena, E.; Russo, V.; Pilato, E.; et al. Inflammation and Cardiovascular Diseases in the Elderly: The Role of Epicardial Adipose Tissue. Front. Med. 2022, 9, 844266. [Google Scholar] [CrossRef]
- Guglielmi, V.; Maresca, L.; D’Adamo, M.; Di Roma, M.; Lanzillo, C.; Federici, M.; Lauro, D.; Preziosi, P.; Bellia, A.; Sbraccia, P. Age-Related Different Relationships between Ectopic Adipose Tissues and Measures of Central Obesity in Sedentary Subjects. PLoS ONE 2014, 9, e103381. [Google Scholar] [CrossRef]
- Karadag, B.; Ozulu, B.; Ozturk, F.Y.; Oztekin, E.; Sener, N.; Altuntas, Y. Comparison of Epicardial Adipose Tissue (EAT) Thickness and Anthropometric Measurements in Metabolic Syndrome (MS) Cases above and under the Age of 65. Arch. Gerontol. Geriatr. 2011, 52, e79–e84. [Google Scholar] [CrossRef]
- Stramaglia, G.; Greco, A.; Guglielmi, G.; De Matthaeis, A.; Vendemiale, G.L. Echocardiography and Dual-Energy X-ray Absorptiometry in the Elderly Patients with Metabolic Syndrome: A Comparison of Two Different Tecniques to Evaluate Visceral Fat Distribution. J. Nutr. Health Aging 2010, 14, 6–10. [Google Scholar] [CrossRef]
- Hell, M.M.; Ding, X.; Rubeaux, M.; Slomka, P.; Gransar, H.; Terzopoulos, D.; Hayes, S.; Marwan, M.; Achenbach, S.; Berman, D.S.; et al. Epicardial Adipose Tissue Volume but Not Density Is an Independent Predictor for Myocardial Ischemia. J. Cardiovasc. Comput. Tomogr. 2016, 10, 141–149. [Google Scholar] [CrossRef]
- Goeller, M.; Achenbach, S.; Marwan, M.; Doris, M.K.; Cadet, S.; Commandeur, F.; Chen, X.; Slomka, P.J.; Gransar, H.; Cao, J.J.; et al. Epicardial Adipose Tissue Density and Volume Are Related to Subclinical Atherosclerosis, Inflammation and Major Adverse Cardiac Events in Asymptomatic Subjects. J. Cardiovasc. Comput. Tomogr. 2018, 12, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Nerlekar, N.; Thakur, U.; Lin, A.; Koh, J.Q.S.; Potter, E.; Liu, D.; Muthalaly, R.G.; Rashid, H.N.; Cameron, J.D.; Dey, D.; et al. The Natural History of Epicardial Adipose Tissue Volume and Attenuation: A Long-Term Prospective Cohort Follow-up Study. Sci. Rep. 2020, 10, 7109. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Harada, K.; Tanaka, A.; Onishi, T.; Matsunaga, S.; Funakubo, H.; Harada, K.; Nagao, T.; Shinoda, N.; Marui, N.; et al. Relationship between Epicardial Adipose Tissue Volume and Coronary Artery Spasm. Int. J. Cardiol. 2021, 324, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lee, W.-H.; Lee, M.-K.; Hsu, P.-C.; Tsai, W.-C.; Chu, C.-Y.; Lee, C.-S.; Yen, H.-W.; Lin, T.-H.; Voon, W.-C.; et al. Epicardial Adipose Tissue Thickness Is Not Associated with Adverse Cardiovascular Events in Patients Undergoing Haemodialysis. Sci. Rep. 2020, 10, 6281. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, R.; Rajani, R.; Cheng, V.Y.; Shmilovich, H.; Nakanishi, R.; Otaki, Y.; Gransar, H.; Slomka, P.J.; Hayes, S.W.; Thomson, L.E.J.; et al. Weight Change Modulates Epicardial Fat Burden: A 4-Year Serial Study with Non-Contrast Computed Tomography. Atherosclerosis 2012, 220, 139–144. [Google Scholar] [CrossRef]
- Fu, C.-P.; Sheu, W.H.-H.; Lee, I.-T.; Tsai, I.-C.; Lee, W.-J.; Liang, K.-W.; Lee, W.-L.; Lin, S.-Y. Effects of Weight Loss on Epicardial Adipose Tissue Thickness and Its Relationship between Serum Soluble CD40 Ligand Levels in Obese Men. Clin. Chim. Acta Int. J. Clin. Chem. 2013, 421, 98–103. [Google Scholar] [CrossRef]
- Willens, H.J.; Byers, P.; Chirinos, J.A.; Labrador, E.; Hare, J.M.; de Marchena, E. Effects of Weight Loss after Bariatric Surgery on Epicardial Fat Measured Using Echocardiography. Am. J. Cardiol. 2007, 99, 1242–1245. [Google Scholar] [CrossRef]
- Kim, M.-K.; Tomita, T.; Kim, M.-J.; Sasai, H.; Maeda, S.; Tanaka, K. Aerobic Exercise Training Reduces Epicardial Fat in Obese Men. J. Appl. Physiol. 2009, 106, 5–11. [Google Scholar] [CrossRef]
- Gaborit, B.; Jacquier, A.; Kober, F.; Abdesselam, I.; Cuisset, T.; Boullu-Ciocca, S.; Emungania, O.; Alessi, M.-C.; Clément, K.; Bernard, M.; et al. Effects of Bariatric Surgery on Cardiac Ectopic Fat: Lesser Decrease in Epicardial Fat Compared to Visceral Fat Loss and No Change in Myocardial Triglyceride Content. J. Am. Coll. Cardiol. 2012, 60, 1381–1389. [Google Scholar] [CrossRef]
- Parisi, V.; Petraglia, L.; D’Esposito, V.; Cabaro, S.; Rengo, G.; Caruso, A.; Grimaldi, M.G.; Baldascino, F.; De Bellis, A.; Vitale, D.; et al. Statin Therapy Modulates Thickness and Inflammatory Profile of Human Epicardial Adipose Tissue. Int. J. Cardiol. 2019, 274, 326–330. [Google Scholar] [CrossRef]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J. Am. Heart Assoc. 2019, 8, e013104. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Melek, B.H.; Arepalli, C.D.; Hartlage, G.-R.; Chen, Z.; Kim, S.; Stillman, A.E.; Raggi, P. Effect of Intensive versus Moderate Lipid-Lowering Therapy on Epicardial Adipose Tissue in Hyperlipidemic Post-Menopausal Women: A Substudy of the BELLES Trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J. Am. Coll. Cardiol. 2013, 61, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Soucek, F.; Covassin, N.; Singh, P.; Ruzek, L.; Kara, T.; Suleiman, M.; Lerman, A.; Koestler, C.; Friedman, P.A.; Lopez-Jimenez, F.; et al. Effects of Atorvastatin (80 Mg) Therapy on Quantity of Epicardial Adipose Tissue in Patients Undergoing Pulmonary Vein Isolation for Atrial Fibrillation. Am. J. Cardiol. 2015, 116, 1443–1446. [Google Scholar] [CrossRef]
- Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; et al. Luseogliflozin Reduces Epicardial Fat Accumulation in Patients with Type 2 Diabetes: A Pilot Study. Cardiovasc. Diabetol. 2017, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Cosson, E.; Nguyen, M.T.; Rezgani, I.; Tatulashvili, S.; Sal, M.; Berkane, N.; Allard, L.; Brillet, P.-Y.; Bihan, H. Epicardial Adipose Tissue Volume and Coronary Calcification among People Living with Diabetes: A Cross-Sectional Study. Cardiovasc. Diabetol. 2021, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Eslami, V.; Baharvand, Z.; Khalili, N.; Saghamanesh, S.; Zarei, E.; Sanei-Taheri, M. Epicardial adipose tissue, inflammatory biomarkers and COVID-19: Is there a possible relationship? Int. Immunopharmacol. 2021, 90, 107174. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Qi, Y.; Deng, L.; Wang, H.; Xu, Y.; Li, Z.; Meng, Z.; Tang, J.; Dai, Z. Obesity as a Potential Predictor of Disease Severity in Young COVID-19 Patients: A Retrospective Study. Obesity 2020, 28, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Cereda, A.; Toselli, M.; Palmisano, A.; Vignale, D.; Khokhar, A.; Campo, G.; Bertini, M.; Loffi, M.; Andreini, D.; Pontone, G.; et al. Coronary calcium score as a predictor of outcomes in the hypertensive Covid-19 population: Results from the Italian (S) Core-Covid-19 Registry. Hypertens. Res. 2022, 45, 333–343. [Google Scholar] [CrossRef]
- Gasecka, A.; Pruc, M.; Kukula, K.; Gilis-Malinowska, N.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, L. Post-COVID-19 heart syndrome. Cardiol. J. 2021, 28, 353–354. [Google Scholar] [CrossRef]
- Ryan, P.M.; Caplice, N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity 2020, 28, 1191–1194. [Google Scholar] [CrossRef]
- Lee, M.-S.; Duan, L.; Clare, R.; Hekimian, A.; Spencer, H.; Chen, W. Comparison of Effects of Statin Use on Mortality in Patients with Heart Failure and Preserved Versus Reduced Left Ventricular Ejection Fraction. Am. J. Cardiol. 2018, 122, 405–412. [Google Scholar] [CrossRef]
- Ahmadi, N.; Hajsadeghi, F.; Nabavi, V.; Arora, R.; Budoff, M. The beneficial effects of statin therapy on epicardial adipose tissue and coronary plaque volumes with vulnerable characteristics measured by computed tomography angiographY. J. Am. Coll. Cardiol. 2013, 61, E1038. [Google Scholar] [CrossRef]
- Yamada, Y.; Takeuchi, S.; Yoneda, M.; Ito, S.; Sano, Y.; Nagasawa, K.; Matsuura, N.; Uchinaka, A.; Murohara, T.; Nagata, K. Atorvastatin Reduces Cardiac and Adipose Tissue Inflammation in Rats with Metabolic Syndrome. Int. J. Cardiol. 2017, 240, 332–338. [Google Scholar] [CrossRef]
- Tawakol, A.; Fayad, Z.A.; Mogg, R.; Alon, A.; Klimas, M.T.; Dansky, H.; Subramanian, S.S.; Abdelbaky, A.; Rudd, J.H.F.; Farkouh, M.E.; et al. Intensification of Statin Therapy Results in a Rapid Reduction in Atherosclerotic Inflammation: Results of a Multicenter Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Feasibility Study. J. Am. Coll. Cardiol. 2013, 62, 909–917. [Google Scholar] [CrossRef]
- Konwerski, M.; Gąsecka, A.; Opolski, G.; Grabowski, M.; Mazurek, T. Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review. Biology 2022, 11, 355. [Google Scholar] [CrossRef]
- Varjabedian, L.; Bourji, M.; Pourafkari, L.; Nader, N.D. Cardioprotection by Metformin: Beneficial Effects Beyond Glucose Reduction. Am. J. Cardiovasc. Drugs 2018, 18, 181–193. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Campbell, H. Comparison of Reducing Epicardial Fat by Exercise, Diet or Bariatric Surgery Weight Loss Strategies: A Systematic Review and Meta-Analysis. Obes. Rev. 2015, 16, 406–415. [Google Scholar] [CrossRef]
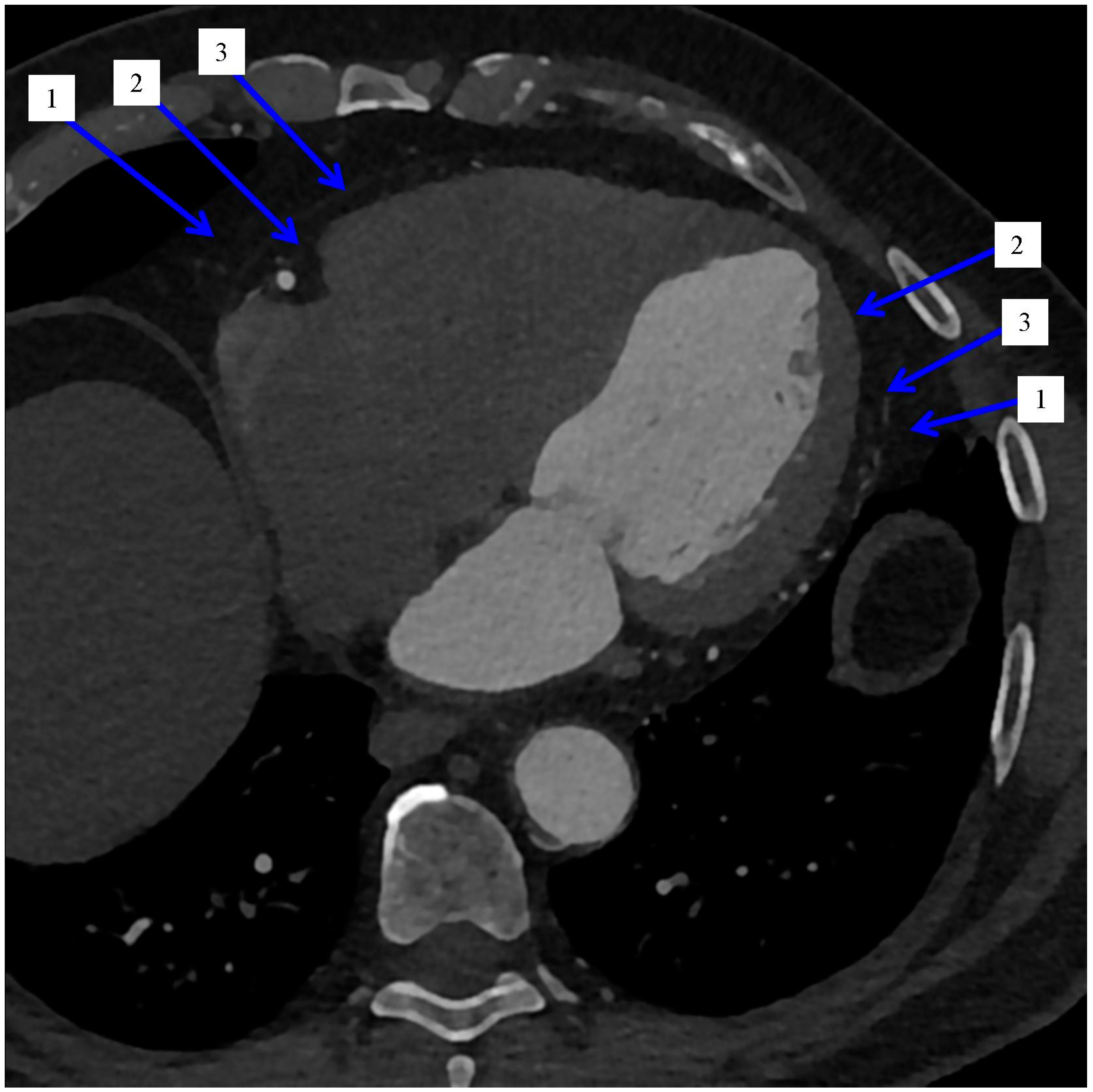
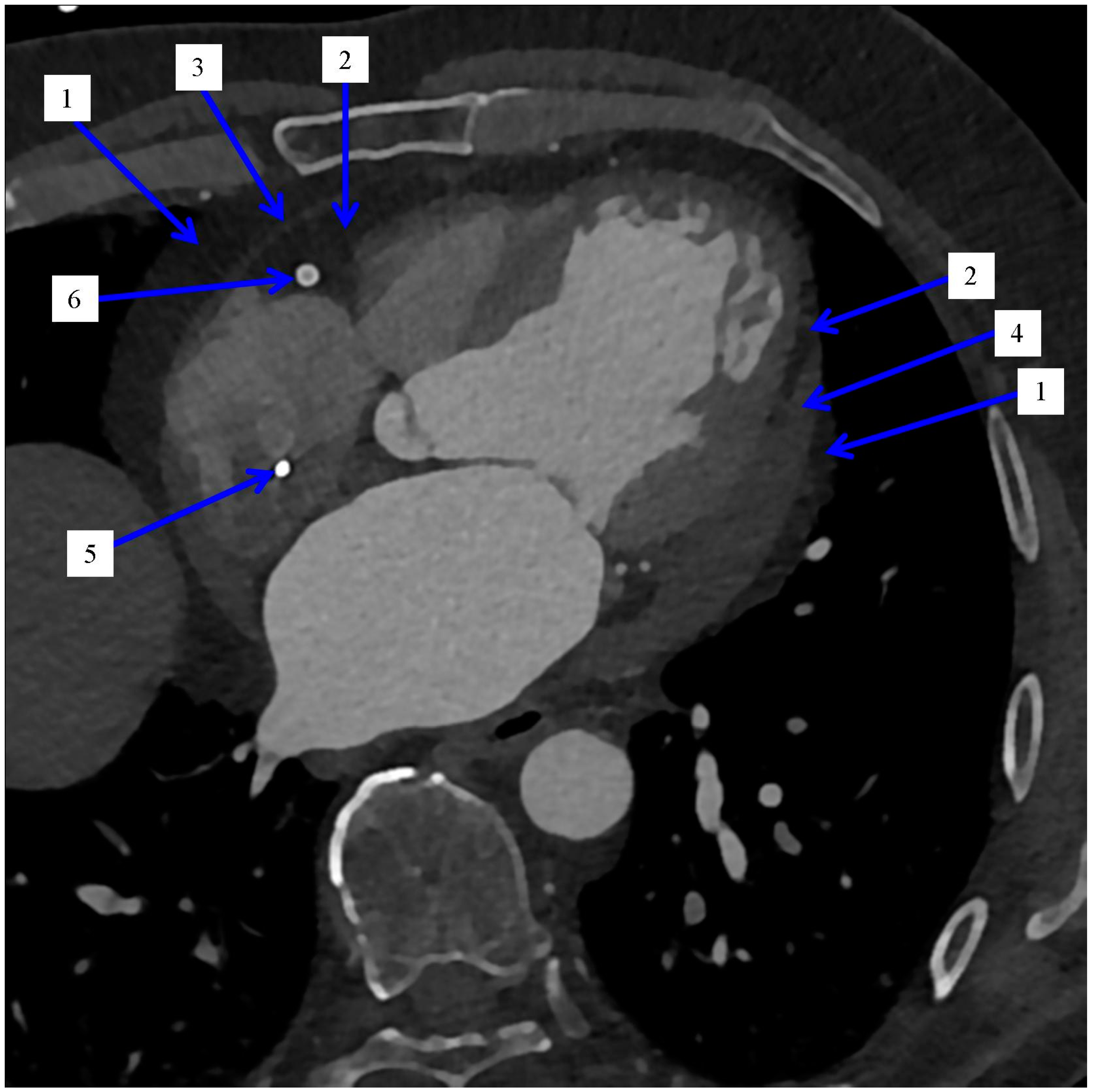
| Refs. | Imaging System | Type of Adipose Tissue | Context of Analyzing Adipose Tissue (Most Important, Based on the Aim of the Study and Conclusions) | Study Population Size |
|---|---|---|---|---|
| Gaborit et al. [6] | MRI | EAT-v | Metabolic risk factors, coronary artery disease | 63 |
| Shmilovich et al. [16] | Non-contrast CT | EAT-v | Predicting major adverse cardiovascular events | 516 |
| Mahabadi et al. [21] | Non-contrast CT | EAT-v | Left atrial size, prevalent and incident atrial fibrillation | 3467 |
| Kaplan et al. [22] | ECHO | EAT-t | Chronic obstructive pulmonary disease, right ventricular systolic dysfunction | 138 (included 40 control subjects) |
| Mahajan et al. [23] | MRI | PeAT | Animal, autopsy pericardial adipose measurements | 10 |
| Saremi et al. [25] | Contrast CT | EAT-v | Regions of heart adipose pockets, comparison with EAT-t | 60 |
| Park et al. [26] | Contrast CT | EAT-v | Threshold-based 3D segmentation, coronary CT angiography | 100 (included 40 control subjects) |
| Ito et al. [29] | Contrast CT | EAT-v | Coronary plaque vulnerability, acute coronary syndrome | 117 |
| Yerramasu et al. [30] | Non-contrast CT | EAT-v | Metabolic syndrome, coronary artery calcium burden, diabetes mellitus | 333 |
| Picard et al. [31] | Contrast CT | EAT-t | Coronary artery disease | 970 |
| Nakanishi et al. [32] | Contrast CT | EAT-v | Coronary artery disease, acute coronary syndrome | 517 |
| Okada et al. [34] | Contrast CT | EAT-v | Coronary artery disease | 140 |
| Demircelik et al. [35] | Contrast CT | EAT-t | Coronary artery disease | 131 |
| Yamashita et al. [36] | Contrast CT | EAT-v | Non-culprit coronary lesions, coronary plaque burden | 54 |
| Alexopoulos et al. [39] | Contrast CT | EAT-v | Coronary artery disease | 214 |
| Uygur et al. [41] | Contrast CT | EAT-v | Coronary artery disease, diabetes mellitus | 157 |
| Greif et al. [42] | CT | PeAT | Coronary artery disease, intermediate pretest likelihood | 286 |
| Janik et al. [43] | Non-contrast CT | EAT-v | Coronary artery disease, intermediate pretest likelihood, ischemic heart disease | 97 |
| Kalaycioglu et al. [44] | ECHO | EAT-t | Chronic obstructive pulmonary disease, systolic pulmonary arterial pressure | 129 |
| Zagaceta et al. [45] | CT | EAT-v | Chronic obstructive pulmonary disease, smoking history, physical activity | 241 |
| Demir et al. [46] | ECHO | EAT-t | Chronic obstructive pulmonary disease, metabolic syndrome, ischemic heart disease | 166 (included 84 control subjects) |
| Kiraz et al. [47] | ECHO | EAT-t | Chronic obstructive pulmonary disease, BODE index | 202 (included 45 control subjects) |
| Ding et al. [48] | CT | PeAT | Coronary artery disease | 998 |
| Unubol et al. [51] | ECHO | EAT-t | Subclinical hypothyroidism | 62 (included 25 control subjects) |
| Sayin et al. [52] | ECHO | EAT-t | Subclinical hypothyroidism | 86 (included 42 control subjects) |
| Korkmaz et al. [53] | ECHO | EAT-t | Subclinical hypothyroidism | 85 (included 24 control subjects) |
| Asik et al. [54] | ECHO | EAT-t | Carotid intima media thickness, Hashimoto thyroiditis, subclinical hypothyroidism | 57 |
| Yazıcı et al. [55] | ECHO | EAT-t | Carotid intima media thickness, subclinical hypothyroidism, restoration of the euthyroid state | 73 (included 30 control subjects) |
| Santos et al. [56] | ECHO | EAT-t | Subclinical hypothyroidism | 100 (included 48 control subjects) |
| Canpolat et al. [62] | ECHO | EAT-t | Atrial fibrillation, ablation | 234 |
| Chao et al. [63] | ECHO | EAT-t | Atrial fibrillation, ablation | 283 |
| Sanghai et al. [64] | Contrast CT | EAT-v | Indexed left atrial epicardial adipose tissue (iLAEAT), atrial fibrillation, ablation | 274 |
| Kawasaki et al. [65] | Contrast CT | EAT-v | Atrial fibrillation, ablation, cardiac sympathetic nerve activity | 64 |
| Guglielmi et al. [67] | MRI | EAT-v | Expansion of intermuscular adipose tissue, sedentary subjects | 32 |
| Karadag et al. [68] | ECHO | EAT-t | Metabolic syndrome, visceral adiposity | 120 |
| Stramaglia et al. [69] | ECHO | EAT-t | Metabolic syndrome, visceral adiposity, hepatic steatosis, risk of malnutrition in the obese elderly | 55 |
| Hell et al. [70] | Non-contrast CT | EAT-v | Epicardial adipose density, pre-test probability, coronary artery disease, SPECT | 213 |
| Goeller et al. [71] | Non-contrast CT | EAT-v | Epicardial adipose density, early atherosclerosis, plaque inflammation, major adverse cardiac events, coronary calcium | 456 |
| Nerlekar et al. [72] | Contrast CT | EAT-v | Epicardial adipose tissue density, non-obstructive coronary artery disease, statin therapy | 90 |
| Kataoka et al. [73] | CT | EAT-v | Coronary artery spasm, total abdominal adipose tissue area, abdominal visceral adipose tissue | 110 |
| Chen et al. [74] | ECHO | EAT-t | Hemodialysis patients, adverse cardiovascular events | 189 |
| Nakazato et al. [75] | Non-contrast CT | EAT-v | Weight change, coronary calcium score | 374 |
| Fu et al. [76] | MRI | EAT-t | Weight change, metabolic syndrome, diabetes mellitus | 57 (included 25 control subjects) |
| Willens et al. [77] | ECHO | EAT-t | Bariatric surgery, metabolic syndrome, abdominal visceral adipose tissue | 23 |
| Kim et al. [78] | ECHO | EAT-t | Effects of exercise training, ventricular epicardial adipose thickness | 24 |
| Gaborit et al. [79] | MRI | EAT-v | Sleep apnea, bariatric surgery, morbid obesity | 23 |
| Parisi et al. [80] | ECHO | EAT-t | Statin therapy, aortic stenosis, cardiac surgery | 193 |
| Raggi et al. [81] | CT | EAT-v | Epicardial adipose tissue attenuation, statin therapy, coronary artery calcium score, postmenopausal women | 420 |
| Alexopoulos et al. [82] | Non-contrast CT | EAT-v | Electron beam CT scans, statin therapy, postmenopausal women | 420 |
| Soucek et al. [83] | Contrast CT | EAT-v | Statin therapy, atrial fibrillation, pulmonary vein isolation | 79 |
| Bouchi et al. [84] | MRI | EAT-v | Luseogliflozin therapy, diabetes mellitus | 19 |
| Cosson et al. [85] | Non-contrast CT | EAT-v | Coronary artery calcification, diabetes mellitus | 409 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheładze, P.; Martuszewski, A.; Poręba, R.; Gać, P. The Importance of the Assessment of Epicardial Adipose Tissue in Scientific Research. J. Clin. Med. 2022, 11, 5621. https://doi.org/10.3390/jcm11195621
Cheładze P, Martuszewski A, Poręba R, Gać P. The Importance of the Assessment of Epicardial Adipose Tissue in Scientific Research. Journal of Clinical Medicine. 2022; 11(19):5621. https://doi.org/10.3390/jcm11195621
Chicago/Turabian StyleCheładze, Przemysław, Adrian Martuszewski, Rafał Poręba, and Paweł Gać. 2022. "The Importance of the Assessment of Epicardial Adipose Tissue in Scientific Research" Journal of Clinical Medicine 11, no. 19: 5621. https://doi.org/10.3390/jcm11195621
APA StyleCheładze, P., Martuszewski, A., Poręba, R., & Gać, P. (2022). The Importance of the Assessment of Epicardial Adipose Tissue in Scientific Research. Journal of Clinical Medicine, 11(19), 5621. https://doi.org/10.3390/jcm11195621








