Practical Model for Residual/Recurrent Cervical Intraepithelial Lesions in Patients with Negative Margins after Cold-Knife Conization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Follow-Up
2.3. Criteria for Residual/Recurrent Disease
2.4. Predictors and Endpoints
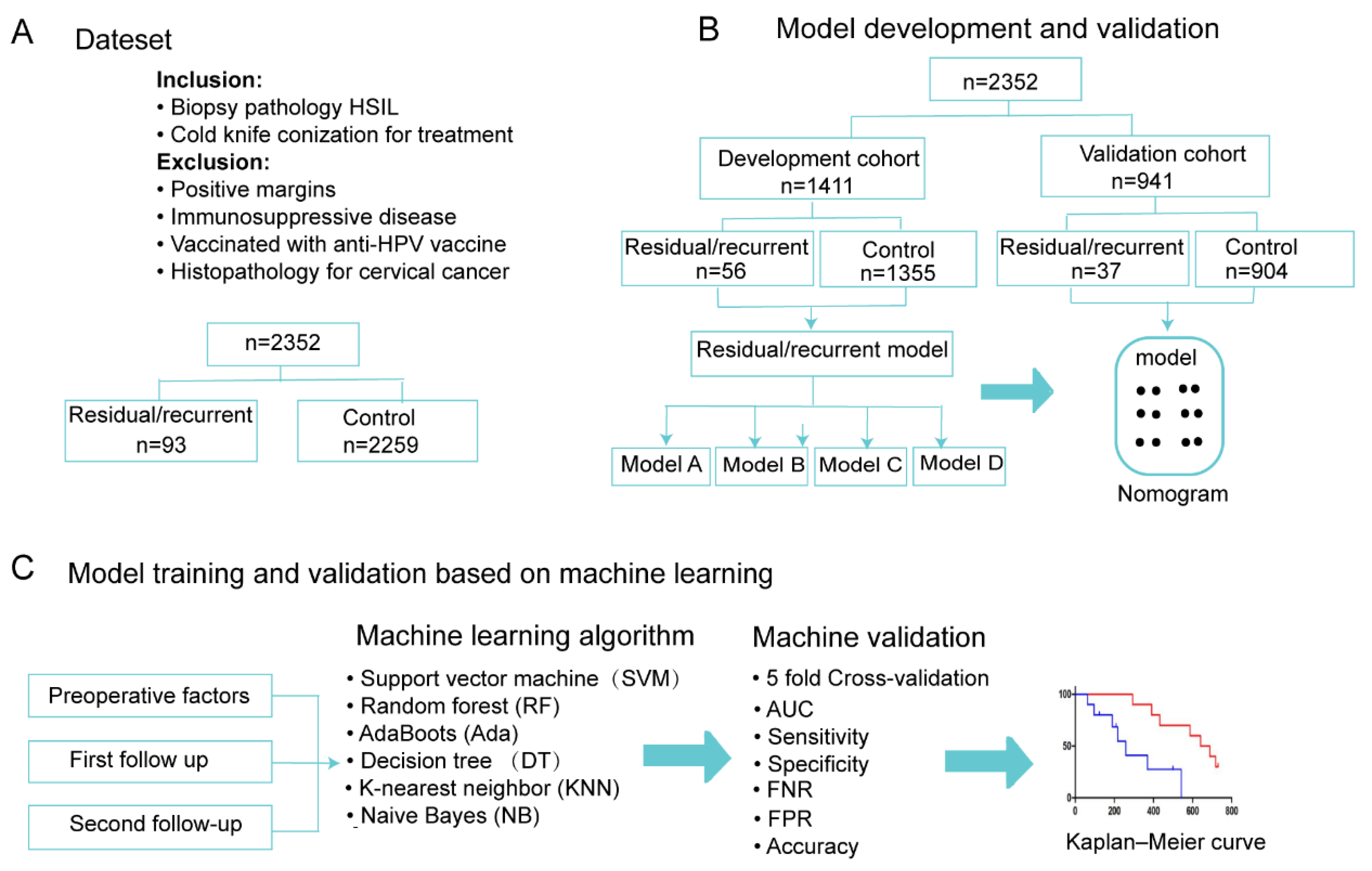
2.5. Model Construction and Validation
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Patients Who Underwent Cold-Knife Conization
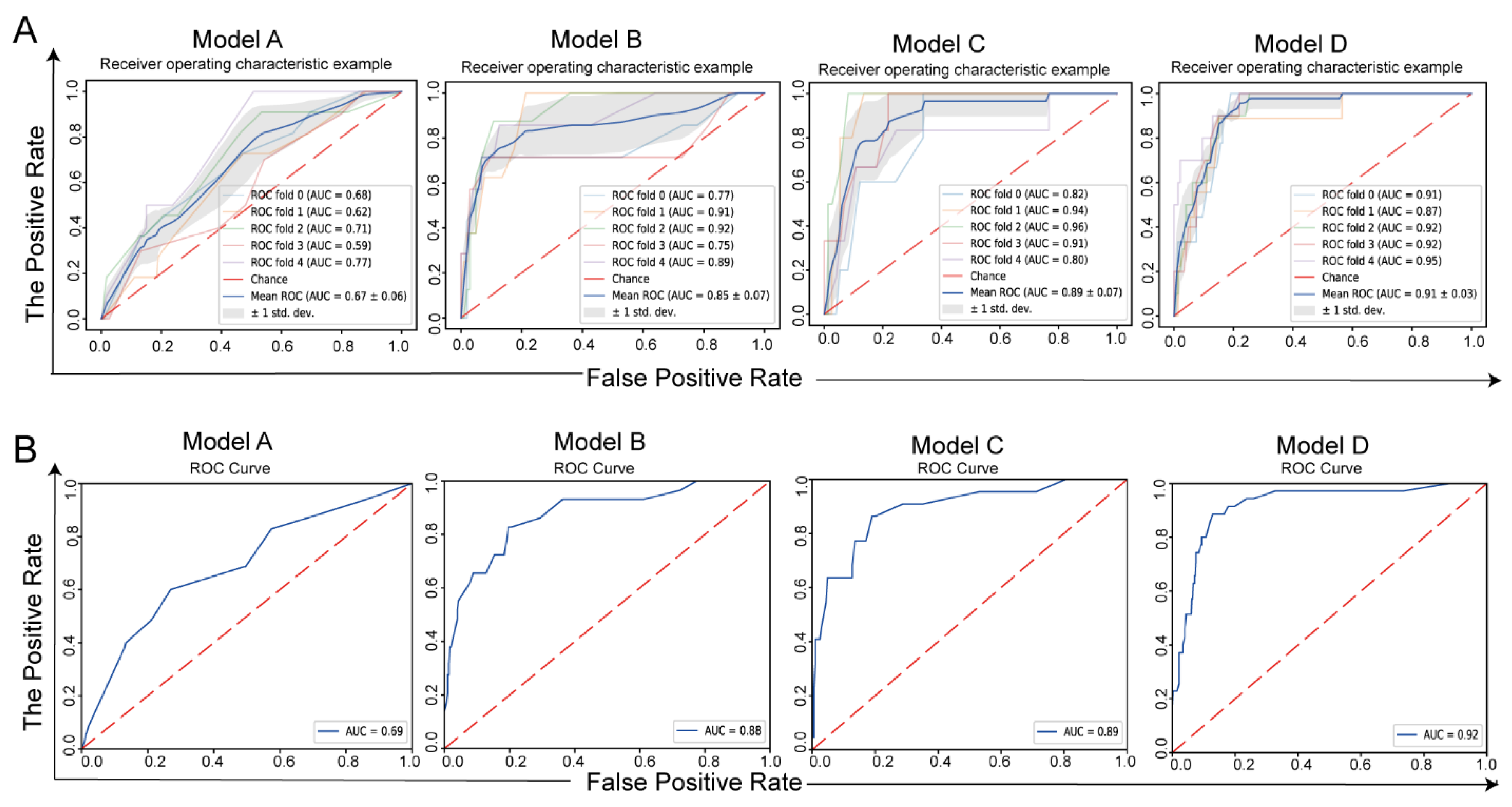
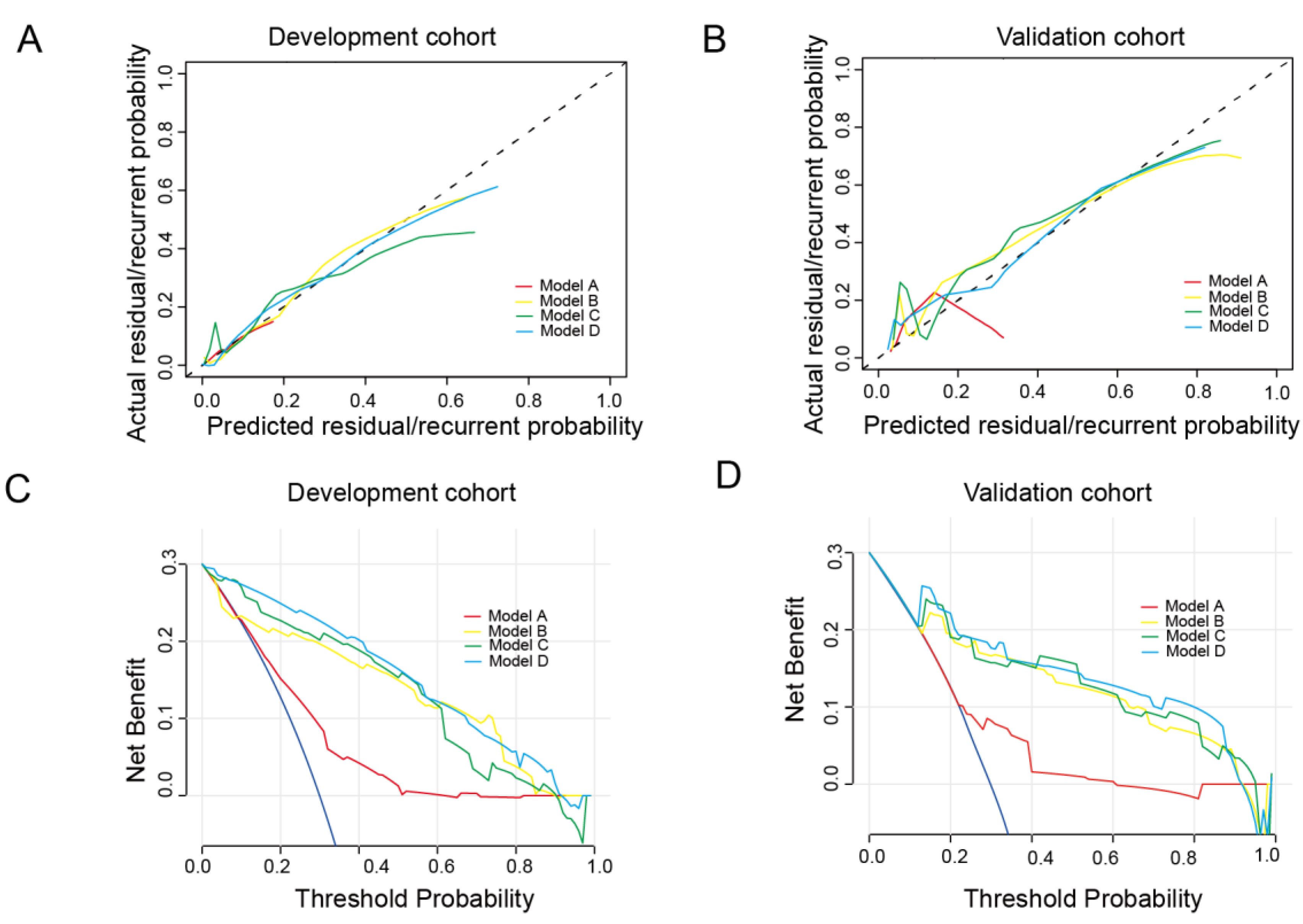
3.2. Model Development and Validation
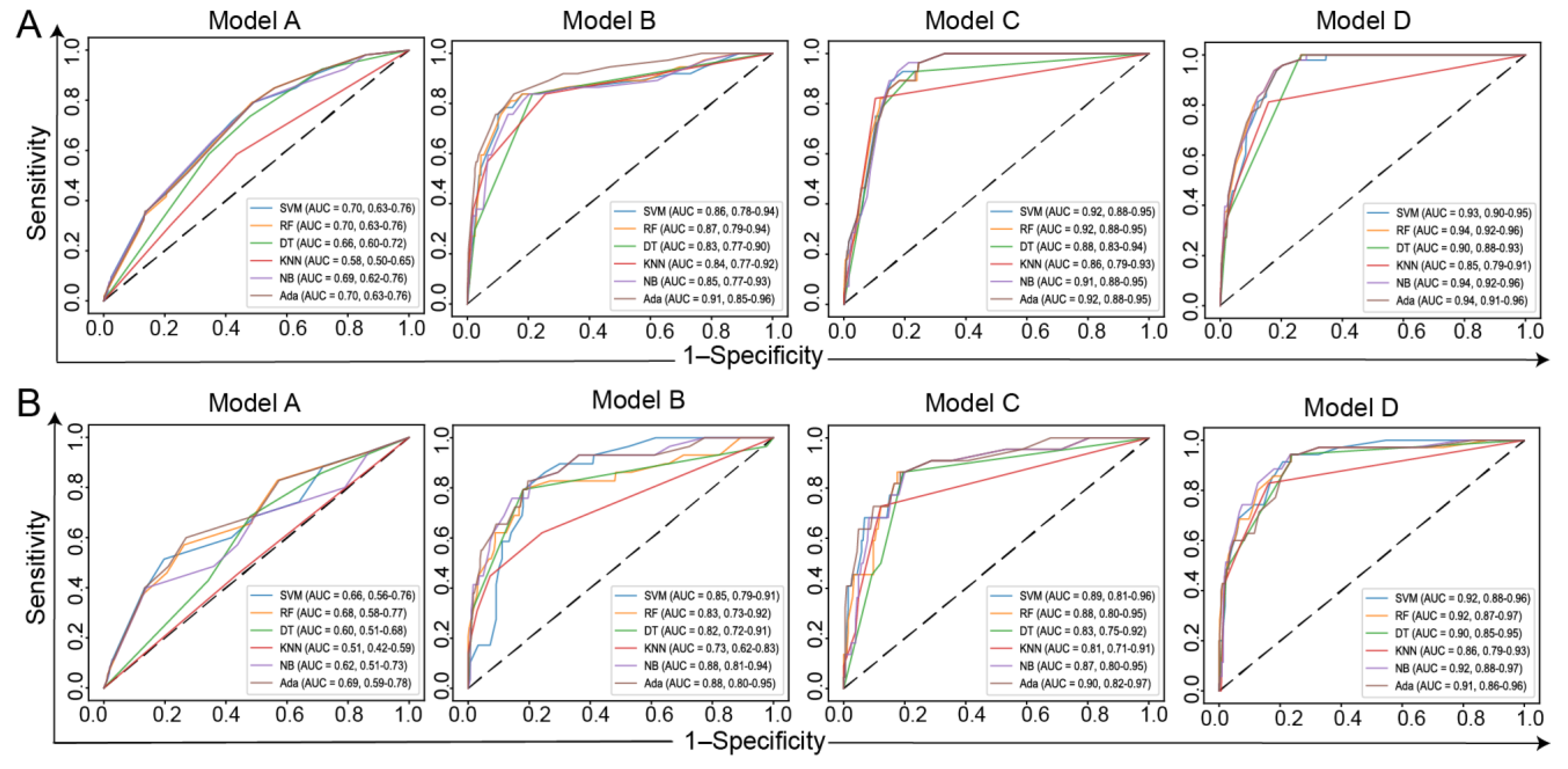
3.3. Model Training and Validation Based on ML Methods
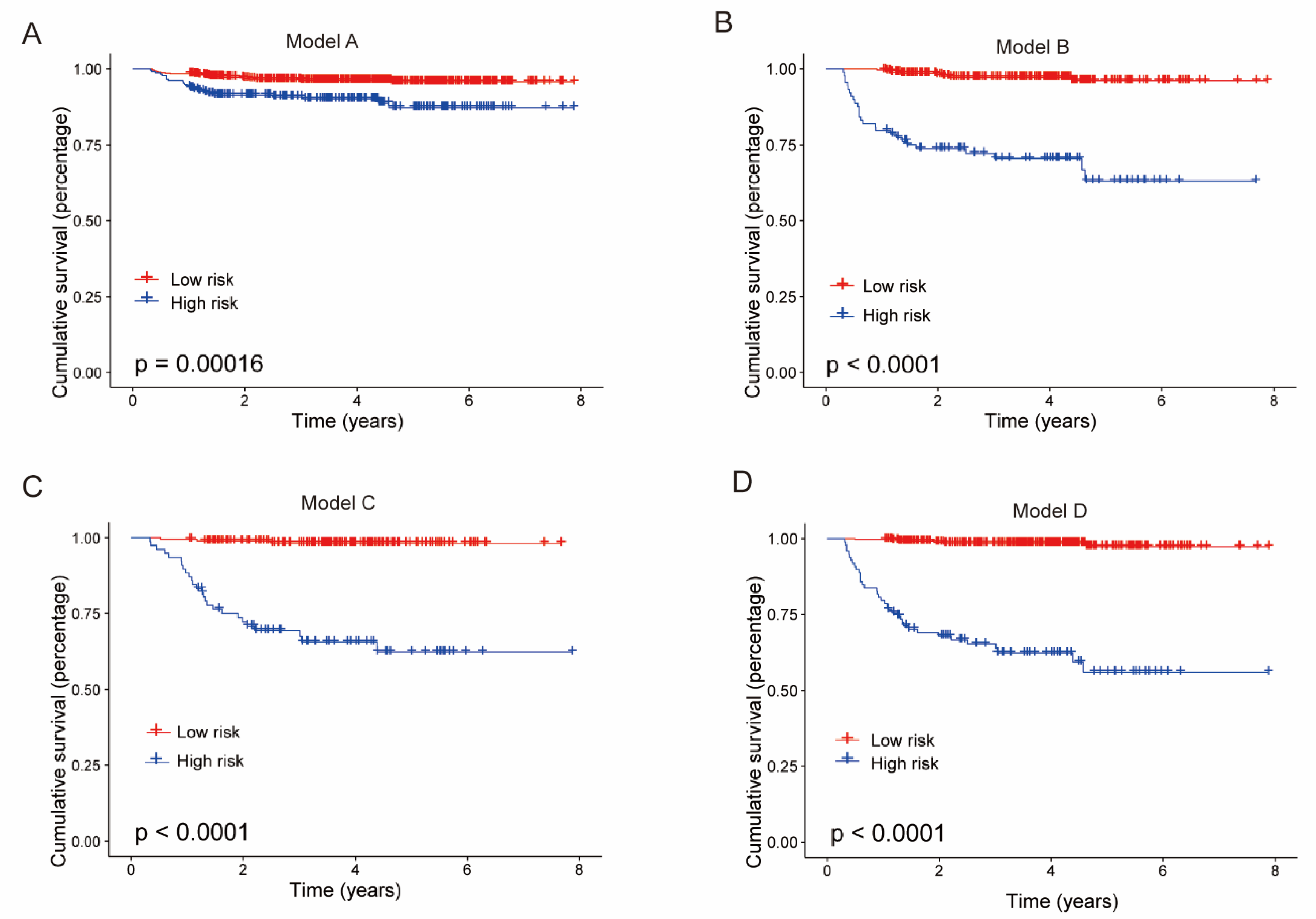
3.4. Kaplan–Meier Estimates
3.5. Model for LEEP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burd, E. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McCredie, M.; Sharples, K.; Paul, C.; Baranyai, J.; Medley, G.; Jones, R.; Skegg, D. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: A retrospective cohort study. Lancet Oncol. 2008, 9, 425–434. [Google Scholar] [CrossRef]
- Perkins, R.; Guido, R.; Castle, P.; Chelmow, D.; Einstein, M.; Garcia, F.; Huh, W.; Kim, J.; Moscicki, A.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012, 30, F88-99. [Google Scholar] [CrossRef]
- Kocken, M.; Helmerhorst, T.J.M.; Berkhof, J.; Louwers, J.A.; Nobbenhuis, M.A.E.; Bais, A.G.; Hogewoning, C.J.A.; Zaal, A.; Verheijen, R.H.M.; Snijders, P.J.F.; et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: A long-term multi-cohort study. Lancet Oncol. 2011, 12, 441–450. [Google Scholar] [CrossRef]
- Cuzick, J.; Clavel, C.; Petry, K.; Meijer, C.; Hoyer, H.; Ratnam, S.; Szarewski, A.; Birembaut, P.; Kulasingam, S.; Sasieni, P.; et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int. J. Cancer 2006, 119, 1095–1101. [Google Scholar] [CrossRef]
- Strander, B.; Andersson-Ellström, A.; Milsom, I.; Sparén, P. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: Population based cohort study. BMJ 2007, 335, 1077. [Google Scholar] [CrossRef] [PubMed]
- Soutter, W.; Sasieni, P.; Panoskaltsis, T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int. J. Cancer 2006, 118, 2048–2055. [Google Scholar] [CrossRef]
- Alder, S.; Megyessi, D.; Sundstrom, K.; Ostensson, E.; Mints, M.; Belkic, K.; Arbyn, M.; Andersson, S. Incomplete excision of cervical intraepithelial neoplasia as a predictor of the risk of recurrent disease-a 16-year follow-up study. Am. J. Obstet. Gynecol. 2020, 222, 172.e1–172.e12. [Google Scholar] [CrossRef]
- Coupe, V.M.; Berkhof, J.; Verheijen, R.H.; Meijer, C.J. Cost-effectiveness of human papillomavirus testing after treatment for cervical intraepithelial neoplasia. BJOG 2007, 114, 416–424. [Google Scholar] [CrossRef]
- Sarian, L.; Derchain, S.; Pitta, D.R.; Morais, S.; Rabelo-Santos, S. Factors associated with HPV persistence after treatment for high-grade cervical intra-epithelial neoplasia with large loop excision of the transformation zone (LLETZ). J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2004, 31, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Prato, B.; Ghelardi, A.; Gadducci, A.; Marchetti, I.; Di Cristofano, C.; Di Coscio, G.; Bevilacqua, G.; Genazzani, A. Correlation of recurrence rates and times with posttreatment human papillomavirus status in patients treated with loop electrosurgical excision procedure conization for cervical squamous intraepithelial lesions. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2008, 18, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.L.; Wang, S.Z.; Chen, H.H.; Huang, Y.X.; Xin, Y.K.; Zhang, T.; Cheng, D.L.; Mao, L.; Li, X.L.; Liu, C.X.; et al. Optimizing the radiomics-machine-learning model based on non-contrast enhanced CT for the simplified risk categorization of thymic epithelial tumors: A large cohort retrospective study. Lung Cancer 2022, 166, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Svetnik, V.; Liaw, A.; Tong, C.; Culberson, J.C.; Sheridan, R.P.; Feuston, B.P. Random forest: A classification and regression tool for compound classification and QSAR modeling. J. Chem. Inf. Comput. Sci. 2003, 43, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Schapire, R.E. Explaining Adaboost. In Empirical Inference; Springer: Berlin/Heidelberg, Germany, 2013; pp. 37–52. [Google Scholar]
- Safavian, S.R.; Landgrebe, D. A survey of decision tree classifier methodology. IEEE Trans. Syst. Man Cybern. 1991, 21, 660–674. [Google Scholar] [CrossRef]
- Peterson, L.E. K-nearest neighbor. Scholarpedia 2009, 4, 1883. [Google Scholar] [CrossRef]
- Rish, I. An Empirical Study of the Naive Bayes Classifier. In Proceedings of the IJCAI 2001 Workshop on Empirical Methods in Artificial Intelligence, New York, NY, USA, 4–6 August 2001; pp. 41–46. [Google Scholar]
- Chu, R.; Chen, W.; Song, G.; Yao, S.; Xie, L.; Song, L.; Zhang, Y.; Chen, L.; Zhang, X.; Ma, Y. Predicting the risk of adverse events in pregnant women with congenital heart disease. J. Am. Heart Assoc. 2020, 9, e016371. [Google Scholar] [CrossRef]
- Yang, F.; Chen, W.; Wei, H.; Zhang, X.; Yuan, S.; Qiao, X.; Chen, Y.-W. Machine learning for histologic subtype classification of non-small cell lung cancer: A retrospective multicenter radiomics study. Front. Oncol. 2021, 10, 608598. [Google Scholar] [CrossRef]
- Gu, Q.; Feng, Z.; Liang, Q.; Li, M.; Deng, J.; Ma, M.; Wang, W.; Liu, J.; Liu, P.; Rong, P. Machine learning-based radiomics strategy for prediction of cell proliferation in non-small cell lung cancer. Eur. J. Radiol. 2019, 118, 32–37. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Darcy, A.; Louie, A.; Roberts, L. Machine Learning and the Profession of Medicine. JAMA 2016, 315, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Montoli, M.E.; Tous, S.; Medina, G.; Castellarnau, M.; Garcia-Tejedor, A.; de Sanjose, S. Long-term predictors of residual or recurrent cervical intraepithelial neoplasia 2-3 after treatment with a large loop excision of the transformation zone: A retrospective study. BJOG 2020, 127, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Cassaro, N.; Garofalo, S.; Boemi, S. HPV16 persistent infection and recurrent disease after LEEP. Virol. J. 2019, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, B.; Chen, L.; Lin, T.; Tong, Y.; Lin, W.; Lin, H.; Gao, Y.; Lin, F.; Sun, P. Evaluation of PCR-Reverse Dot Blot Human Papillomavirus Genotyping Test in Predicting Residual/Recurrent CIN 2+ in Posttreatment Patients in China. Cancer Manag. Res. 2020, 12, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Elfgren, K.; Jacobs, M.; Walboomers, J.; Meijer, C.; Dillner, J. Rate of human papillomavirus clearance after treatment of cervical intraepithelial neoplasia. Obstet. Gynecol. 2002, 100, 965–971. [Google Scholar] [CrossRef]
- Alonso, I.; Torné, A.; Puig-Tintoré, L.M.; Esteve, R.; Quintó, L.; Campo, E.; Pahisa, J.; Ordi, J. Pre- and post-conization high-risk HPV testing predicts residual/recurrent disease in patients treated for CIN 2-3. Gynecol. Oncol. 2006, 103, 631–636. [Google Scholar] [CrossRef]
- Heymans, J.; Benoy, I.; Poppe, W.; Depuydt, C. Type-specific HPV geno-typing improves detection of recurrent high-grade cervical neoplasia after conisation. Int. J. Cancer 2011, 129, 903–909. [Google Scholar] [CrossRef]
- Gök, M.; Coupé, V.M.; Berkhof, J.; Verheijen, R.H.; Helmerhorst, T.J.; Hogewoning, C.J.; Snijders, P.J.; Meijer, C.J. HPV16 and increased risk of recurrence after treatment for CIN. Gynecol. Oncol. 2007, 104, 273–275. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Hur, S.; Cho, C.; Kim, Y.; Kim, S.; Kang, S. Clearance of human papillomavirus infection after successful conization in patients with cervical intraepithelial neoplasia. Int. J. Cancer 2010, 126, 1903–1909. [Google Scholar] [CrossRef]
- Cuello, M.; Espinosa, M.; Orlandini, E.; Hwang, D. The value of endocervical curettage during loop electrosurgical excision procedures in predicting persistent/recurrent preinvasive cervical disease. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2018, 141, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Cervical carcinoma and reproductive factors: Collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int. J. Cancer 2006, 119, 1108–1124. [CrossRef] [PubMed]
- Kitchener, H.; Walker, P.; Nelson, L.; Hadwin, R.; Patnick, J.; Anthony, G.; Sargent, A.; Wood, J.; Moore, C.; Cruickshank, M. HPV testing as an adjunct to cytology in the follow up of women treated for cervical intraepithelial neoplasia. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Houfflin Debarge, V.; Collinet, P.; Vinatier, D.; Ego, A.; Dewilde, A.; Boman, F.; Leroy, J. Value of human papillomavirus testing after conization by loop electrosurgical excision for high-grade squamous intraepithelial lesions. Gynecol. Oncol. 2003, 90, 587–592. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Gao, Y.; Qu, P. Necessity for subsequent surgery in women of child-bearing age with positive margins after conization. BMC Women’s Health 2021, 21, 191. [Google Scholar] [CrossRef]

| Development Cohort (1411) | Validation Cohort (941) | |||||
|---|---|---|---|---|---|---|
| Patient Characteristic | No Residual/ | Residual/ | p-Value | No Residual/ | Residual/ | p-Value |
| Recurrent CIN (1355) | Recurrent CIN (56) | Recurrent CIN (904) | Recurrent CIN (37) | |||
| Age (years) | ||||||
| <45 | 1004 | 44 | 0.552 | 659 | 26 | 0.87 |
| ≥45 | 351 | 12 | 245 | 11 | ||
| Pregnancy | ||||||
| <3 | 904 | 27 | 0.007 | 609 | 13 | <0.001 |
| ≥3 | 451 | 29 | 295 | 24 | ||
| Parity | ||||||
| <2 | 808 | 32 | 0.823 | 539 | 19 | 0.408 |
| ≥2 | 547 | 24 | 365 | 18 | ||
| Menopause | ||||||
| Yes | 1288 | 50 | 0.109 | 858 | 35 | 1 |
| No | 67 | 6 | 46 | 2 | ||
| TCT | ||||||
| <ASCUS | 307 | 11 | 0.544 | 226 | 14 | 0.222 |
| ≥ASCUS | 922 | 43 | 580 | 22 | ||
| Unknown | 126 | 2 | 98 | 1 | ||
| HPV16/18 or RLUs > 1000 | ||||||
| Yes | 625 | 38 | 0.024 | 341 | 13 | 0.477 |
| No | 512 | 15 | 421 | 22 | ||
| Unknown | 218 | 3 | 142 | 2 | ||
| Transformation zone | ||||||
| Type I/II | 632 | 29 | 0.492 | 409 | 22 | 0.882 |
| Type III | 102 | 7 | 75 | 5 | ||
| Unknown | 621 | 20 | 420 | 10 | ||
| ECC | ||||||
| Positive | 36 | 5 | 0.02 | 27 | 5 | 0.003 |
| Negative | 1319 | 51 | 877 | 32 | ||
| Improved | ||||||
| Yes | 565 | 14 | 0.019 | 369 | 14 | 0.849 |
| No | 790 | 42 | 535 | 23 | ||
| Severe | ||||||
| No | 1056 | 39 | 0.195 | 706 | 29 | 1 |
| Yes | 299 | 17 | 198 | 8 | ||
| First follow-up after conization | ||||||
| HR-HPV negative | 585 | 7 | <0.001 | 382 | 7 | <0.001 |
| HR-HPV positive | 166 | 36 | 103 | 25 | ||
| Unknown | 604 | 13 | 419 | 5 | ||
| TCT < ASCUS | 611 | 27 | <0.001 | 378 | 20 | <0.001 |
| TCT >= ASCUS | 16 | 13 | 6 | 9 | ||
| Unknown | 728 | 16 | 520 | 8 | ||
| Second follow-up after conization | ||||||
| HR-HPV negative | 384 | 4 | <0.001 | 242 | 5 | <0.001 |
| HR-HPV positive | 114 | 32 | 53 | 20 | ||
| Unknown | 857 | 20 | ||||
| TCT < ASCUS | 435 | 23 | <0.001 | 261 | 15 | <0.001 |
| TCT > ASCUS | 13 | 7 | 7 | 10 | ||
| Unknown | 907 | 26 | 636 | 12 | ||
| Threshold | AUC | Sensitivity | Specificity | FPR | FNR | Accuracy | |
|---|---|---|---|---|---|---|---|
| Development-Cohort Cross-Validation | |||||||
| Model A | 0.51 ± 0.12 | 0.58 ± 0.13 | 0.73 ± 0.24 | 0.58 ± 0.15 | 0.4 ± 0.15 | 0.2 ± 0.24 | 0.58 ± 0.13 |
| Model B | 0.65 ± 0.15 | 0.85 ± 0.07 | 0.88 ± 0.17 | 0.86 ± 0.09 | 0.14 ± 0.09 | 0.1 ± 0.17 | 0.86 ± 0.08 |
| Model C | 0.51 ± 0.24 | 0.89 ± 0.07 | 0.97 ± 0.07 | 0.80 ± 0.09 | 0.20 ± 0.09 | 0.0 ± 0.07 | 0.81 ± 0.08 |
| Model D | 0.59 ± 0.15 | 0.92 ± 0.01 | 0.94 ± 0.12 | 0.84 ± 0.06 | 0.1 ± 0.06 | 0.0 ± 0.12 | 0.85 ± 0.05 |
| Validation cohort | |||||||
| Model A | 0.53 | 0.69 (0.59–0.78) | 0.60 | 0.73 | 0.27 | 0.4 | 0.72 |
| Model B | 0.44 | 0.88 (0.80–0.95) | 0.83 | 0.80 | 0.20 | 0.17 | 0.81 |
| Model C | 0.43 | 0.89 (0.81–0.97) | 0.86 | 0.81 | 0.19 | 0.14 | 0.82 |
| Model D | 0.42 | 0.91 (0.87–0.96) | 0.94 | 0.77 | 0.23 | 0.06 | 0.78 |
| AUC | Sensitivity | Specificity | FPR | FNR | Accuracy | |
|---|---|---|---|---|---|---|
| Model A | ||||||
| SVM | 0.66 (0.56–0.76) | 0.51 | 0.80 | 0.20 | 0.49 | 0.79 |
| RF | 0.68 (0.58–0.77) | 0.57 | 0.74 | 0.26 | 0.43 | 0.73 |
| DT | 0.60 (0.51–0.68) | 0.69 | 0.52 | 0.48 | 0.31 | 0.53 |
| KNN | 0.51 (0.42–0.59) | 0.46 | 0.56 | 0.44 | 0.54 | 0.56 |
| NB | 0.62 (0.51–0.73) | 0.4 | 0.86 | 0.14 | 0.6 | 0.84 |
| Ada | 0.69 (0.59–0.78) | 0.6 | 0.73 | 0.27 | 0.4 | 0.73 |
| Model B | ||||||
| SVM | 0.85 (0.79–0.91) | 0.79 | 0.82 | 0.18 | 0.21 | 0.82 |
| RF | 0.83 (0.73–0.92) | 0.79 | 0.82 | 0.18 | 0.21 | 0.82 |
| DT | 0.82 (0.72–0.91) | 0.79 | 0.82 | 0.18 | 0.21 | 0.82 |
| KNN | 0.73 (0.62–0.83) | 0.62 | 0.76 | 0.24 | 0.38 | 0.75 |
| NB | 0.88 (0.81–0.94) | 0.83 | 0.80 | 0.21 | 0.17 | 0.80 |
| Ada | 0.88 (0.80–0.95) | 0.83 | 0.80 | 0.20 | 0.17 | 0.81 |
| Model C | ||||||
| SVM | 0.89 (0.81–0.96) | 0.86 | 0.80 | 0.20 | 0.14 | 0.81 |
| RF | 0.88 (0.80–0.95) | 0.86 | 0.82 | 0.18 | 0.14 | 0.83 |
| DT | 0.83 (0.75–0.92) | 0.86 | 0.80 | 0.20 | 0.14 | 0.81 |
| KNN | 0.81 (0.71–0.91) | 0.72 | 0.88 | 0.12 | 0.27 | 0.86 |
| NB | 0.87 (0.80–0.95) | 0.86 | 0.80 | 0.2 | 0.14 | 0.81 |
| Ada | 0.90 (0.82–0.97) | 0.86 | 0.81 | 0.19 | 0.14 | 0.82 |
| Model D | ||||||
| SVM | 0.91 (0.87–0.96) | 0.94 | 0.77 | 0.24 | 0.06 | 0.78 |
| RF | 0.92 (0.87–0.96) | 0.94 | 0.77 | 0.23 | 0.06 | 0.78 |
| DT | 0.90 (0.85–0.95) | 0.94 | 0.76 | 0.24 | 0.06 | 0.78 |
| KNN | 0.86 (0.79–0.93) | 0.8 | 0.86 | 0.14 | 0.2 | 0.86 |
| NB | 0.92 (0.88–0.97) | 0.94 | 0.77 | 0.23 | 0.06 | 0.78 |
| Ada | 0.91 (0.86–0.96) | 0.94 | 0.77 | 0.23 | 0.06 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Dong, Y.; Liu, L.; Jia, L.; Meng, L.; Liu, H.; Wang, L.; Xu, Y.; Zhang, Y.; Qiao, X. Practical Model for Residual/Recurrent Cervical Intraepithelial Lesions in Patients with Negative Margins after Cold-Knife Conization. J. Clin. Med. 2022, 11, 5634. https://doi.org/10.3390/jcm11195634
Chen W, Dong Y, Liu L, Jia L, Meng L, Liu H, Wang L, Xu Y, Zhang Y, Qiao X. Practical Model for Residual/Recurrent Cervical Intraepithelial Lesions in Patients with Negative Margins after Cold-Knife Conization. Journal of Clinical Medicine. 2022; 11(19):5634. https://doi.org/10.3390/jcm11195634
Chicago/Turabian StyleChen, Wei, Yajie Dong, Lu Liu, Lin Jia, Lihua Meng, Hongli Liu, Lili Wang, Ying Xu, Youzhong Zhang, and Xu Qiao. 2022. "Practical Model for Residual/Recurrent Cervical Intraepithelial Lesions in Patients with Negative Margins after Cold-Knife Conization" Journal of Clinical Medicine 11, no. 19: 5634. https://doi.org/10.3390/jcm11195634







