The Atypical Manifestation of Pulmonary Tuberculosis in Patients with Bronchial Anthracofibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CT Protocol
2.3. Image Analysis
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Patients
3.2. Clinical Features and Microbiology Results of Pulmonary TB According to the Presence of BAF
3.3. CT Findings of Pulmonary TB According to the Presence of BAF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, M.P.; Lee, K.S.; Han, J.; Kim, H.; Rhee, C.H.; Han, Y.C.; Kwon, O.J. Bronchial stenosis due to anthracofibrosis. Chest 1998, 113, 344–350. [Google Scholar] [CrossRef]
- Shah, A.; Kunal, S.; Gothi, R. Bronchial anthracofibrosis: The spectrum of radiological appearances. Indian J. Radiol. Imaging 2018, 28, 333–341. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.H.; Im, S.A.; Kim, Y.K.; Lee, K.Y. CT differentiation of anthracofibrosis from endobronchial tuberculosis. AJR Am. J. Roentgenol. 2008, 191, 247–251. [Google Scholar] [CrossRef]
- Kim, H.Y.; Im, J.G.; Goo, J.M.; Kim, J.Y.; Han, S.K.; Lee, J.K.; Song, J.W. Bronchial anthracofibrosis (inflammatory bronchial stenosis with anthracotic pigmentation): CT findings. AJR Am. J. Roentgenol. 2000, 174, 523–527. [Google Scholar] [CrossRef]
- Ko, J.M.; Park, H.J. Main pulmonary artery dilatation in patients with anthracofibrosis. J. Korean Med. Sci. 2014, 29, 1577–1582. [Google Scholar] [CrossRef]
- Hwang, J.; Puttagunta, L.; Green, F.; Shimanovsky, A.; Barrie, J.; Long, R. Bronchial anthracofibrosis and tuberculosis in immigrants to Canada from the Indian subcontinent. Int. J. Tuberc. Lung Dis. 2010, 14, 231–237. [Google Scholar]
- Kim, Y.J.; Jung, C.Y.; Shin, H.W.; Lee, B.K. Biomass smoke induced bronchial anthracofibrosis: Presenting features and clinical course. Respir. Med. 2009, 103, 757–765. [Google Scholar] [CrossRef]
- Najafizadeh, K.; Ghorbani, F.; Farnia, P.; Shiehmorteza, M.; Jamali, M. Spoligotyping of Mycobacterium tuberculosis in anthracotic bronchitis. Int. J. Tuberc. Lung Dis. 2008, 12, 962–966. [Google Scholar]
- Kala, J.; Sahay, S.; Shah, A. Bronchial anthracofibrosis and tuberculosis presenting as a middle lobe syndrome. Prim. Care Respir. J. 2008, 17, 51–55. [Google Scholar] [CrossRef]
- Wynn, G.J.; Turkington, P.M.; O’Driscoll, B.R. Anthracofibrosis, bronchial stenosis with overlying anthracotic mucosa: Possibly a new occupational lung disorder: A series of seven cases From one UK hospital. Chest 2008, 134, 1069–1073. [Google Scholar] [CrossRef]
- Naccache, J.M.; Monnet, I.; Nunes, H.; Billon-Galland, M.A.; Pairon, J.C.; Guillon, F.; Valeyre, D. Anthracofibrosis attributed to mixed mineral dust exposure: Report of three cases. Thorax 2008, 63, 655–657. [Google Scholar] [CrossRef]
- Jamaati, H.; Sharifi, A.; Mirenayat, M.S.; Mirsadraee, M.; Amoli, K.; Heidarnazhad, H.; Sigari, N.; Saeedfar, K.; Kahkouee, S.; Pourabdollah Toutkaboni, M.; et al. What Do We Know about Anthracofibrosis? A Literature Review. Tanaffos 2017, 16, 175–189. [Google Scholar]
- Im, J.G.; Itoh, H.; Lee, K.S.; Han, M.C. CT-pathology correlation of pulmonary tuberculosis. Crit. Rev. Diagn. Imaging 1995, 36, 227–285. [Google Scholar]
- Lee, K.S.; Song, K.S.; Lim, T.H.; Kim, P.N.; Kim, I.Y.; Lee, B.H. Adult-onset pulmonary tuberculosis: Findings on chest radiographs and CT scans. AJR Am. J. Roentgenol. 1993, 160, 753–758. [Google Scholar] [CrossRef]
- Lee, K.S.; Im, J.G. CT in adults with tuberculosis of the chest: Characteristic findings and role in management. AJR Am. J. Roentgenol. 1995, 164, 1361–1367. [Google Scholar] [CrossRef]
- Ko, J.M.; Park, H.J.; Kim, C.H.; Song, S.W. The relation between CT findings and sputum microbiology studies in active pulmonary tuberculosis. Eur. J. Radiol. 2015, 84, 2339–2344. [Google Scholar] [CrossRef]
- Im, J.G.; Itoh, H.; Shim, Y.S.; Lee, J.H.; Ahn, J.; Han, M.C.; Noma, S. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology 1993, 186, 653–660. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Lee, K.S. Pulmonary tuberculosis: Up-to-date imaging and management. AJR Am. J. Roentgenol. 2008, 191, 834–844. [Google Scholar] [CrossRef]
- Ikezoe, J.; Takeuchi, N.; Johkoh, T.; Kohno, N.; Tomiyama, N.; Kozuka, T.; Noma, K.; Ueda, E. CT appearance of pulmonary tuberculosis in diabetic and immunocompromised patients: Comparison with patients who had no underlying disease. AJR Am. J. Roentgenol. 1992, 159, 1175–1179. [Google Scholar] [CrossRef]
- Chung, M.J.; Goo, J.M.; Im, J.G. Pulmonary tuberculosis in patients with idiopathic pulmonary fibrosis. Eur. J. Radiol. 2004, 52, 175–179. [Google Scholar] [CrossRef]
- Kim, H.Y.; Im, J.G.; Goo, J.M.; Lee, J.K.; Song, J.W.; Kim, S.K. Pulmonary tuberculosis in patients with systematic lupus erythematosus. AJR Am. J. Roentgenol. 1999, 173, 1639–1642. [Google Scholar] [CrossRef]
- Gruden, J.F.; Webb, W.R.; Naidich, D.P.; McGuinness, G. Multinodular disease: Anatomic localization at thin-section CT—Multireader evaluation of a simple algorithm. Radiology 1999, 210, 711–720. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, T.S.; Han, J.; Hwang, J.H.; Yoon, J.H.; Kim, Y.; Yoo, S.Y. Diffuse micronodular lung disease: HRCT and pathologic findings. J. Comput. Assist. Tomogr. 1999, 23, 99–106. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Muller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Hatipoglu, O.N.; Osma, E.; Manisali, M.; Ucan, E.S.; Balci, P.; Akkoclu, A.; Akpinar, O.; Karlikaya, C.; Yuksel, C. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax 1996, 51, 397–402. [Google Scholar] [CrossRef]
- Lee, K.S.; Hwang, J.W.; Chung, M.P.; Kim, H.; Kwon, O.J. Utility of CT in the evaluation of pulmonary tuberculosis in patients without AIDS. Chest 1996, 110, 977–984. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, Y.H.; Kim, W.S.; Hwang, S.H.; Kim, P.N.; Lee, B.H. Endobronchial tuberculosis: CT features. J. Comput. Assist. Tomogr. 1991, 15, 424–428. [Google Scholar] [CrossRef]
- No, T.-M.; Kim, I.-S.; Kim, S.W.; Park, D.-H.; Joeng, J.-K.; Ju, D.; Chyun, J.-H.; Kim, Y.-J.; Shin, H.W.; Lee, B.K. The clinical investigation for determining the etiology of bronchial anthracofibrosis. Korean J. Intern. Med. 2003, 65, 665–674. [Google Scholar]
- Sandoval, J.; Salas, J.; Martinez-Guerra, M.L.; Gómez, A.; Martinez, C.; Portales, A.; Palomar, A.; Villegas, M.; Barrios, R. Pulmonary arterial hypertension and cor pulmonale associated with chronic domestic woodsmoke inhalation. Chest 1993, 103, 12–20. [Google Scholar] [CrossRef]
- Gold, J.A.; Jagirdar, J.; Hay, J.G.; Addrizzo-Harris, D.J.; Naidich, D.P.; Rom, W.N. Hut lung. A domestically acquired particulate lung disease. Medicine 2000, 79, 310–317. [Google Scholar] [CrossRef]
- Choe, H.; Lee, I.; Lee, Y. The CT Findings of Bronchial Anthracofibrosis: Comparison of Cases with or without Active Tuberculosis. J. Korean Radiol. Soc. 2004, 50, 109–114. [Google Scholar] [CrossRef]
- Long, R.; Wong, E.; Barrie, J. Bronchial anthracofibrosis and tuberculosis: CT features before and after treatment. AJR Am. J. Roentgenol. 2005, 184, S33–S36. [Google Scholar] [CrossRef]
- Kahkouee, S.; Khabbaz, S.S.; Keshavarz, E.; Kiani, A.; Hajinasrollah, G.; Anvar, A.G. Diagnostic triad of pulmonary anthracofibrosis in spiral CT scan—A retrospective study. Pol. J. Radiol. 2019, 84, e234–e239. [Google Scholar] [CrossRef] [PubMed]
- Kahkouee, S.; Pourghorban, R.; Bitarafan, M.; Najafizadeh, K.; Makki, S.S. Imaging Findings of Isolated Bronchial Anthracofibrosis: A Computed Tomography Analysis of Patients With Bronchoscopic and Histologic Confirmation. Arch. Bronconeumol. 2015, 51, 322–327. [Google Scholar] [CrossRef]
- Gonlugur, U.; Gonlugur, T.; Ozer, S. Middle Lobe Syndrome Associated with Bronchial Anthracofibrosis. Acta Med. Litu. 2020, 27, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Pilaniya, V.; Kunal, S.; Shah, A. Occurrence of bronchial anthracofibrosis in respiratory symptomatics with exposure to biomass fuel smoke. Adv. Respir. Med. 2017, 85, 127–135. [Google Scholar] [CrossRef]
- Kashyap, S.; Solanki, A. Challenges in endobronchial tuberculosis: From diagnosis to management. Pulm. Med. 2014, 2014, 594806. [Google Scholar] [CrossRef] [PubMed]
- Smart, J. Endo-bronchial tuberculosis. Br. J. Tuberc. Dis. Chest 1951, 45, 61–68. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.D.; Shin, D.W.; Bae, S.H.; Kim, A.L.; Kim, J.N.; Jung, S.W.; Lee, B.K.; Kim, Y.J. Relationship between bronchial anthracofibrosis and endobronchial tuberculosis. Korean J. Intern. Med. 2013, 28, 330–338. [Google Scholar] [CrossRef]
- Zelikoff, J.T.; Chen, L.C.; Cohen, M.D.; Schlesinger, R.B. The toxicology of inhaled woodsmoke. J. Toxicol. Environ. Health B Crit. Rev. 2002, 5, 269–282. [Google Scholar] [CrossRef]
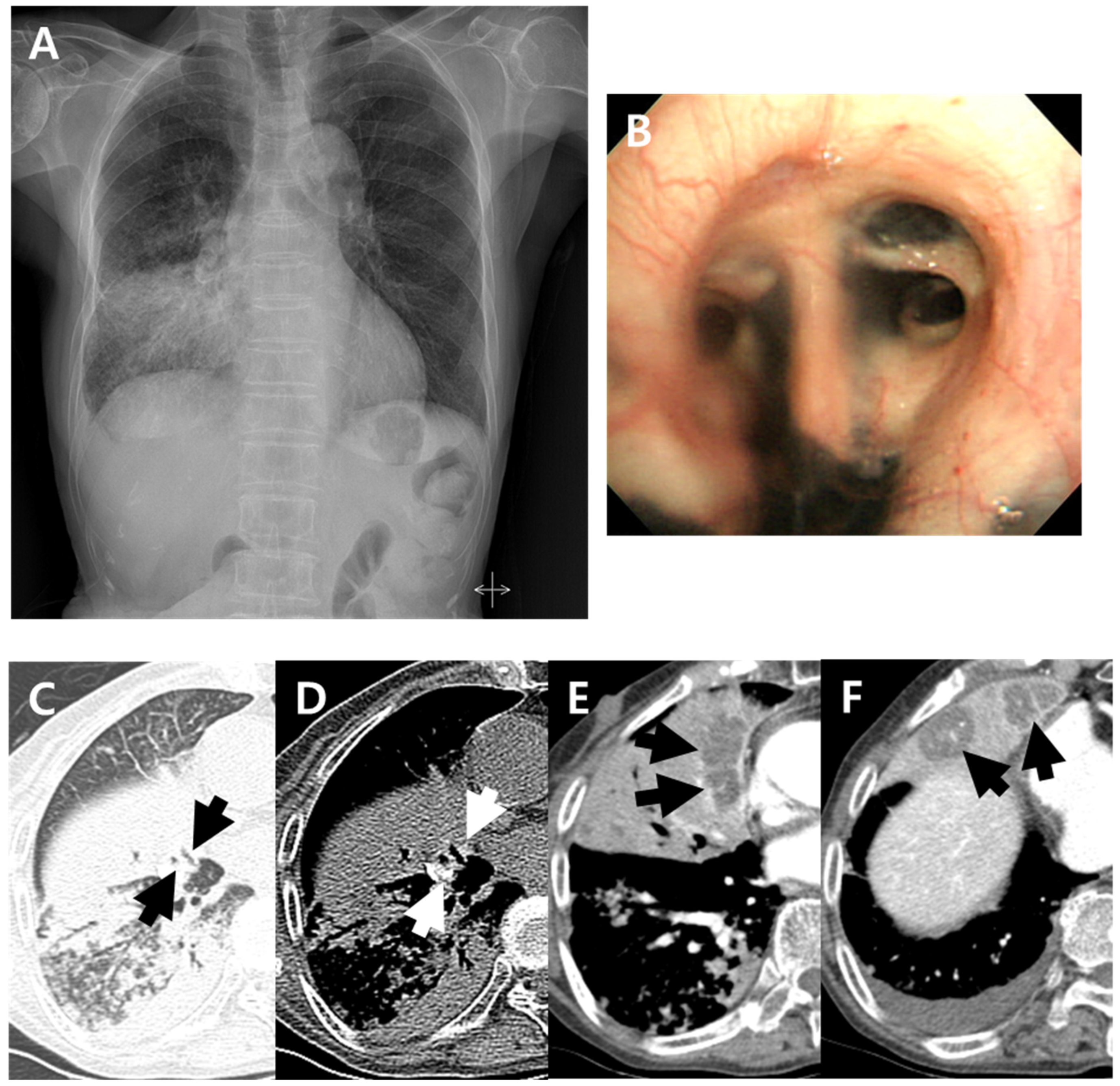

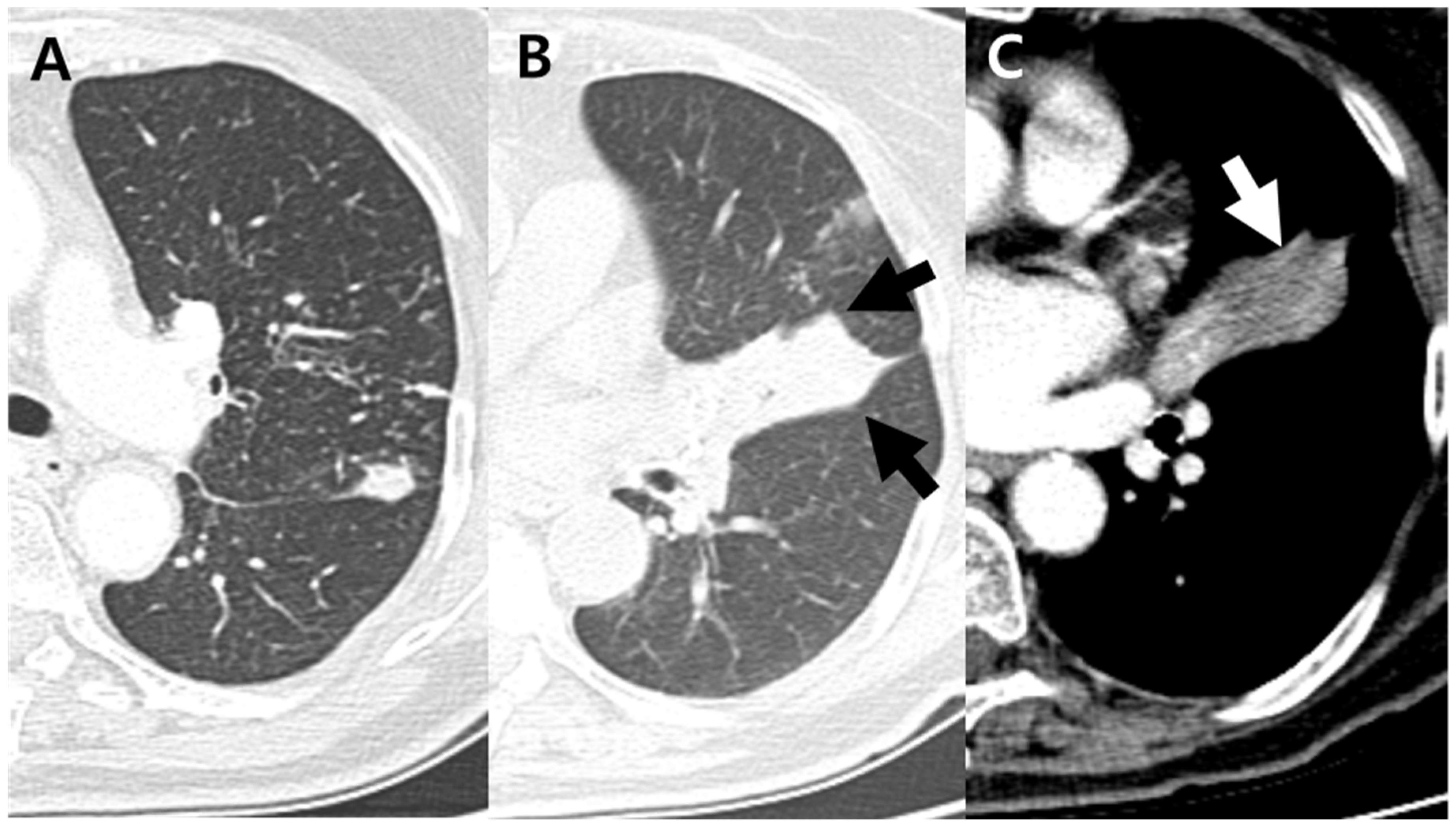
| Total | Anthracofibrosis Group | Nonanthracofibrosis Group | p Value a | |
|---|---|---|---|---|
| Number of patients | 202 | 28 | 174 | |
| Age (mean ± SD, range) | 59 ± 18 (16–95) | 79 ± 7 (64–94) | 56 ± 17 (16–95) | <0.001 ***b |
| Sex (M:F) | 126:76 | 3:25 | 123:51 | <0.001 *** |
| Smoking history, M:F | 95 (47%), 90:5 | 2 (7%), 2:0 | 93 (53%), 88:5 | <0.001 *** |
| Co-morbidity | 97 (48%) | 11 (39%) | 86 (49%) | 0.319 |
| Chronic renal failure | 4 (2%) | 1 (4%) | 3 (2%) | 0.452 |
| Malignancy | 17(8%) | 4 (14%) | 13(7%) | 0.264 |
| AIDS | 1 (0.5%) | 0 | 1 (0.6%) | 1.000 |
| DM | 58(29%) | 6 (21%) | 52(30%) | 0.359 |
| Collagen vascular disease | 5 (2%) | 0 | 5 (3%) | 1.000 |
| COPD, asthma | 5 (2%) | 1 (4%) | 4 (2%) | 0.530 |
| Liver disease | 17 (8%) | 0 | 17 (10%) | 0.137 |
| Sputum | ||||
| AFB positivity | 49/130 (38%) | 6/18 (33%) | 43/112 (38%) | 0.681 |
| AFB number, mean ± SD | 0.86 ± 1.26 | 0.56 ± 0.86 | 0.91 ± 1.31 | 0.143 b |
| PCR positivity | 60/111 (54%) | 8/17 (47%) | 52/94 (55%) | 0.529 |
| Culture positivity | 73/115 (63%) | 11/15 (73%) | 62/100 (62%) | 0.395 |
| Bronchial washing fluid | ||||
| AFB positivity | 68/198 (34%) | 12/27 (44%) | 56/171 (33%) | 0.234 |
| AFB number, mean ± SD | 0.73 ± 1.12 | 0.78 ± 1.01 | 0.73 ± 1.14 | 0.822 b |
| PCR positivity | 153/199 (77%) | 27/28 (96%) | 126/171 (74%) | 0.008 ** |
| Culture positivity | 177/200 (88%) | 27/28 (96%) | 150/172 (87%) | 0.211 |
| Total (n = 202) | Anthracofibrosis Group (n = 28) | Nonanthracofibrosis Group (n = 174) | p Value c | |
|---|---|---|---|---|
| Micronodule | 187 (93%) | 25 (89%) | 162 (93%) | 0.443 |
| Centrilobular | 106 (52%) | 18 (64%) | 88 (51%) | 0.178 |
| Perilymphatic a | 169 (84%) | 21 (75%) | 148 (85%) | 0.179 |
| Peribronchovascular | 166 (82%) | 21 (75%) | 145 (83%) | 0.292 |
| Septal | 103 (51%) | 10 (36%) | 93 (53%) | 0.081 |
| Subpleural | 56 (28%) | 9 (32%) | 47 (27%) | 0.573 |
| Random | 11 (5%) | 2 (7%) | 9 (5%) | 0.652 |
| Tree-in-bud | 86 (43%) | 15 (54%) | 71 (41%) | 0.205 |
| Consolidation/Macronodule | 183 (91%) | 25 (89%) | 158 (91%) | 0.732 |
| Cavitation | 93 (46%) | 4 (14%) | 89 (51%) | 0.001 ** |
| Ground glass opacity | 39 (19%) | 8 (29%) | 31 (18%) | 0.181 |
| Bronchovascular bundle thickening | 157(78%) | 19 (68%) | 138(79%) | 0.176 |
| Interlobular septal thickening | 138 (68%) | 16 (57%) | 122 (70%) | 0.171 |
| Atoll sign | 4 (2%) | 0 | 4 (2%) | 1.000 |
| Galaxy/Cluster sign | 14 (7%) | 0 | 14 (8%) | 0.225 |
| Lymphadenopathy | 65 (32%) | 16 (57%) | 49 (28%) | 0.002 ** |
| Endobronchial involvement b | 39 (19%) | 13 (46%) | 26 (15%) | <0.001 *** |
| Internal low-density area or focal contour bulge within atelectasis | 20 (10%) | 18 (64%) | 2 (1%) | <0.001 *** |
| Upper lobe predominance | 150 (74%) | 9 (32%) | 141 (81%) | <0.001 *** |
| Lower lobe predominance | 27 (13%) | 12 (43%) | 15 (9%) | <0.001 *** |
| No zonal predominance | 25 (12%) | 7 (25%) | 18 (10%) | 0.056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, M.K.; Lee, S.Y.; Ko, J.M. The Atypical Manifestation of Pulmonary Tuberculosis in Patients with Bronchial Anthracofibrosis. J. Clin. Med. 2022, 11, 5646. https://doi.org/10.3390/jcm11195646
Jung MK, Lee SY, Ko JM. The Atypical Manifestation of Pulmonary Tuberculosis in Patients with Bronchial Anthracofibrosis. Journal of Clinical Medicine. 2022; 11(19):5646. https://doi.org/10.3390/jcm11195646
Chicago/Turabian StyleJung, Min Kyung, Sang Young Lee, and Jeong Min Ko. 2022. "The Atypical Manifestation of Pulmonary Tuberculosis in Patients with Bronchial Anthracofibrosis" Journal of Clinical Medicine 11, no. 19: 5646. https://doi.org/10.3390/jcm11195646
APA StyleJung, M. K., Lee, S. Y., & Ko, J. M. (2022). The Atypical Manifestation of Pulmonary Tuberculosis in Patients with Bronchial Anthracofibrosis. Journal of Clinical Medicine, 11(19), 5646. https://doi.org/10.3390/jcm11195646






