Inflammatory Bowel Disease: Role of Vagus Nerve Stimulation
Abstract
1. Introduction
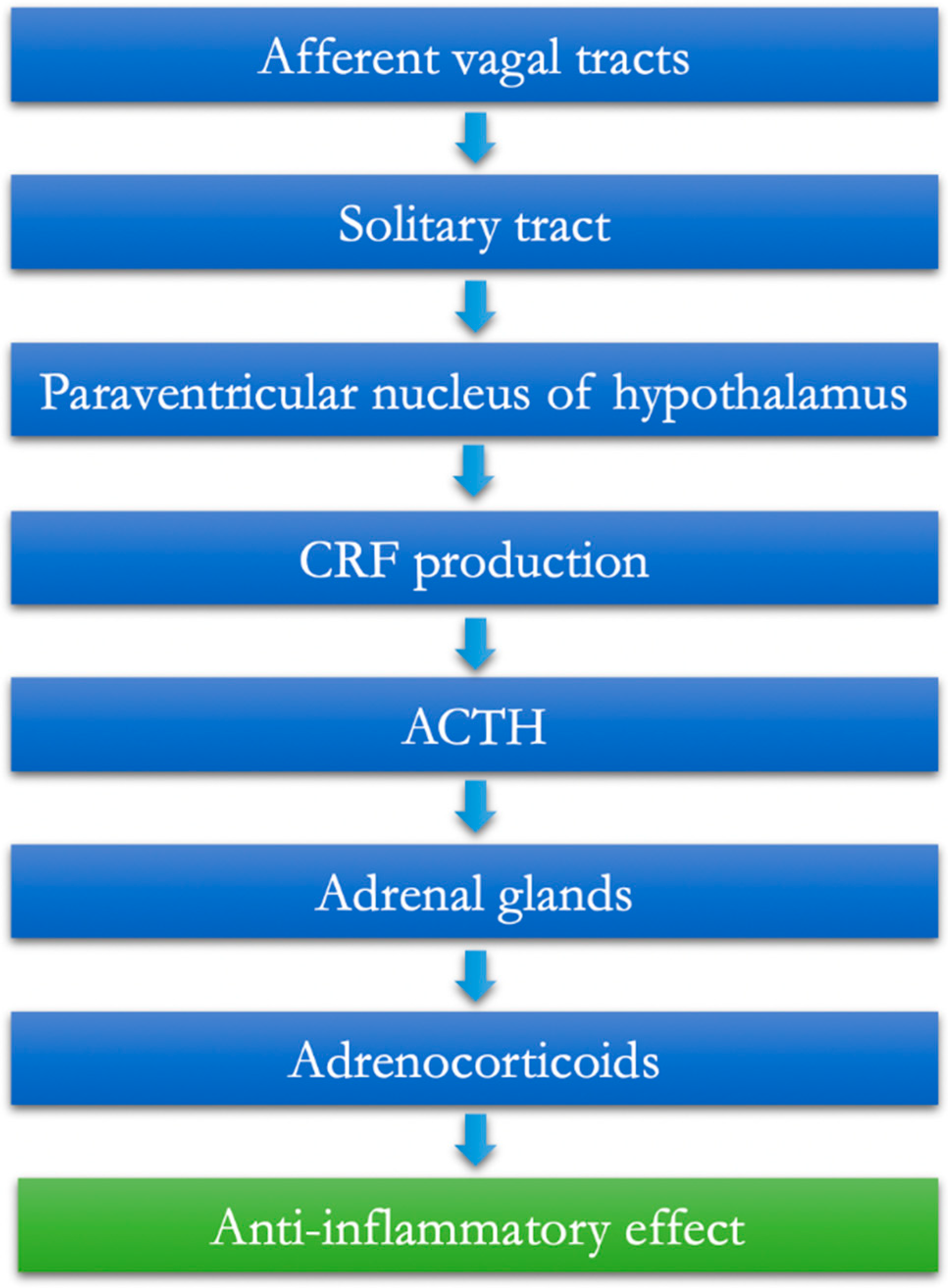
2. The Afferent Vagus and the Hypothalamic–Pituitary–Adrenal Anti-Inflammatory Pathway
- The vagal afferents are equipped with IL-1β receptors at the paraganglia level [6].
- From here, the information is conveyed to the core of the solitary tract.
- Through the paraventricular nucleus of the hypothalamus, the information is then extended to populations of specific neurons that release CRF (corticotropin-releasing factor) [7].
- The final task of these neurons is, therefore, to favor the release of the pituitaryadrenocorticotropic hormone (ACTH), which, as known, can, in turn, mediate the production and release of adrenocorticoids from the adrenal gland, having established anti-inflammatory effects (hypothalamic–pituitary–adrenal axis, HPA) (Figure 1).
The Vagus Nerve at the Microbiota–Gut–Brain Axis Interconnection
3. The Anti-Inflammatory Cholinergic Vagal Pathway (CAIP: Cholinergic Anti-Inflammatory Pathway)
4. Possible Role of Vagus Nerve Stimulation in Chronic Inflammatory Bowel Disease
4.1. Implantation of the Vagal Stimulator
4.2. Clinical Data in Inflammatory Bowel Disease
4.3. Possible Inclusion Criteria of Future Studies
- CD with extensive small bowel disease or less than 200 cm of small bowel due to surgery and refractory to all target therapies;
- CD with contraindications to target therapies (fragile patients, history of cancer, history of severe infections);
- Patients with CD who do not want to undergo target therapy or with a history of poor adherence to therapy;
- Steroid-dependent UC.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Prechtl, J.C.; Powley, T.L. The Fiber Composition of the Abdominal Vagus of the Rat. Anat. Embryol. 1990, 181, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Berthoud, H.R.; Stead, R.H. Vagal Afferent Nerve Fibres Contact Mast Cells in Rat Small Intestinal Mucosa. Neuroimmunomodulation 1997, 4, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Powley, T.L.; Jaffey, D.M.; McAdams, J.; Baronowsky, E.A.; Black, D.; Chesney, L.; Evans, C.; Phillips, R.J. Vagal Innervation of the Stomach Reassessed: Brain-Gut Connectome Uses Smart Terminals. Ann. N. Y. Acad. Sci. 2019, 1454, 14–30. [Google Scholar] [CrossRef]
- Goehler, L.E.; Relton, J.K.; Dripps, D.; Kiechle, R.; Tartaglia, N.; Maier, S.F.; Watkins, L.R. Vagal Paraganglia Bind Biotinylated Interleukin-1 Receptor Antagonist: A Possible Mechanism for Immune-to-Brain Communication. Brain Res. Bull. 1997, 43, 357–364. [Google Scholar] [CrossRef]
- Rivest, S.; Lacroix, S.; Vallières, L.; Nadeau, S.; Zhang, J.; Laflamme, N. How the Blood Talks to the Brain Parenchyma and the Paraventricular Nucleus of the Hypothalamus during Systemic Inflammatory and Infectious Stimuli. Proc. Soc. Exp. Biol. Med. 2000, 223, 22–38. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Caviglia, G.P.; Abdulle, A.; Pellicano, R.; Ditto, M.C.; Morino, M.; Fusaro, E.; Saracco, G.M.; Bugianesi, E.; Astegiano, M. Adalimumab Therapy Improves Intestinal Dysbiosis in Crohn’s Disease. J. Clin. Med. 2019, 8, 1646. [Google Scholar] [CrossRef] [PubMed]
- Raybould, H.E. Gut Chemosensing: Interactions between Gut Endocrine Cells and Visceral Afferents. Auton. Neurosci. 2010, 153, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tanida, M.; Yamano, T.; Maeda, K.; Okumura, N.; Fukushima, Y.; Nagai, K. Effects of Intraduodenal Injection of Lactobacillus Johnsonii La1 on Renal Sympathetic Nerve Activity and Blood Pressure in Urethane-Anesthetized Rats. Neurosci. Lett. 2005, 389, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic Acetylcholine Receptor Alpha7 Subunit Is an Essential Regulator of Inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Fornai, M.; van den Wijngaard, R.M.; Antonioli, L.; Pellegrini, C.; Blandizzi, C.; de Jonge, W.J. Neuronal Regulation of Intestinal Immune Functions in Health and Disease. Neurogastroenterol. Motil. 2018, 30, e13406. [Google Scholar] [CrossRef]
- Cui, W.-Y.; Li, M.D. Nicotinic Modulation of Innate Immune Pathways via A7 Nicotinic Acetylcholine Receptor. J. Neuroimmune Pharm. 2010, 5, 479–488. [Google Scholar] [CrossRef]
- Liu, B.; Wanders, A.; Wirdefeldt, K.; Sjölander, A.; Sachs, M.C.; Eberhardson, M.; Ye, W.; Ekbom, A.; Olén, O.; Ludvigsson, J.F. Vagotomy and Subsequent Risk of Inflammatory Bowel Disease: A Nationwide Register-Based Matched Cohort Study. Aliment. Pharm. 2020, 51, 1022–1030. [Google Scholar] [CrossRef]
- Olofsson, P.S.; Katz, D.A.; Rosas-Ballina, M.; Levine, Y.A.; Ochani, M.; Valdés-Ferrer, S.I.; Pavlov, V.A.; Tracey, K.J.; Chavan, S.S. A7 Nicotinic Acetylcholine Receptor (A7nAChR) Expression in Bone Marrow-Derived Non-T Cells Is Required for the Inflammatory Reflex. Mol. Med. 2012, 18, 539–543. [Google Scholar] [CrossRef]
- Martelli, D.; McKinley, M.J.; McAllen, R.M. The Cholinergic Anti-Inflammatory Pathway: A Critical Review. Auton. Neurosci. 2014, 182, 65–69. [Google Scholar] [CrossRef]
- Okabe, Y.; Medzhitov, R. Tissue-Specific Signals Control Reversible Program of Localization and Functional Polarization of Macrophages. Cell 2014, 157, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-Immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Niess, J.H.; Brand, S.; Gu, X.; Landsman, L.; Jung, S.; McCormick, B.A.; Vyas, J.M.; Boes, M.; Ploegh, H.L.; Fox, J.G.; et al. CX3CR1-Mediated Dendritic Cell Access to the Intestinal Lumen and Bacterial Clearance. Science 2005, 307, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Mowat, A.M. Macrophages in Intestinal Homeostasis and Inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3+ Regulatory T Cells in the Lamina Propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef]
- Rivollier, A.; He, J.; Kole, A.; Valatas, V.; Kelsall, B.L. Inflammation Switches the Differentiation Program of Ly6Chi Monocytes from Antiinflammatory Macrophages to Inflammatory Dendritic Cells in the Colon. J. Exp. Med. 2012, 209, 139–155. [Google Scholar] [CrossRef]
- Matteoli, G.; Gomez-Pinilla, P.J.; Nemethova, A.; Di Giovangiulio, M.; Cailotto, C.; van Bree, S.H.; Michel, K.; Tracey, K.J.; Schemann, M.; Boesmans, W.; et al. A Distinct Vagal Anti-Inflammatory Pathway Modulates Intestinal Muscularis Resident Macrophages Independent of the Spleen. Gut 2014, 63, 938–948. [Google Scholar] [CrossRef]
- Muller, P.A.; Koscsó, B.; Rajani, G.M.; Stevanovic, K.; Berres, M.-L.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Li, X.-M.; Mucida, D.; et al. Crosstalk between Muscularis Macrophages and Enteric Neurons Regulates Gastrointestinal Motility. Cell 2014, 158, 1210. [Google Scholar] [CrossRef]
- Ghia, J.-E.; Park, A.J.; Blennerhassett, P.; Khan, W.I.; Collins, S.M. Adoptive Transfer of Macrophage from Mice with Depression-like Behavior Enhances Susceptibility to Colitis. Inflamm. Bowel. Dis. 2011, 17, 1474–1489. [Google Scholar] [CrossRef]
- Cailotto, C.; Gomez-Pinilla, P.J.; Costes, L.M.; van der Vliet, J.; Di Giovangiulio, M.; Némethova, A.; Matteoli, G.; Boeckxstaens, G.E. Neuro-Anatomical Evidence Indicating Indirect Modulation of Macrophages by Vagal Efferents in the Intestine but Not in the Spleen. PLoS ONE 2014, 9, e87785. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Olofsson, P.S.; Tracey, K.J. Bioelectronic Medicine: Technology Targeting Molecular Mechanisms for Therapy. J. Intern. Med. 2017, 282, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.E.; Morsi, A.; Tanweer, O.; Grobelny, B.; Geller, E.; Carlson, C.; Devinsky, O.; Doyle, W.K. Efficacy of Vagus Nerve Stimulation over Time: Review of 65 Consecutive Patients with Treatment-Resistant Epilepsy Treated with VNS > 10 Years. Epilepsy. Behav. 2011, 20, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, S.; Lilja, B.; Rosén, I.; Sundkvist, G. Disturbed Autonomic Nerve Function in Patients with Crohn’s Disease. Scand. J. Gastroenterol. 1991, 26, 361–366. [Google Scholar] [CrossRef]
- Lindgren, S.; Stewenius, J.; Sjölund, K.; Lilja, B.; Sundkvist, G. Autonomic Vagal Nerve Dysfunction in Patients with Ulcerative Colitis. Scand. J. Gastroenterol. 1993, 28, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, S.; Dantzer, C.; Mondillon, L.; Trocme, C.; Gauchez, A.-S.; Ducros, V.; Mathieu, N.; Toussaint, B.; Fournier, A.; Canini, F.; et al. Relationship between Vagal Tone, Cortisol, TNF-Alpha, Epinephrine and Negative Affects in Crohn’s Disease and Irritable Bowel Syndrome. PLoS ONE 2014, 9, e105328. [Google Scholar] [CrossRef] [PubMed]
- Mikocka-Walus, A.; Knowles, S.R.; Keefer, L.; Graff, L. Controversies Revisited: A Systematic Review of the Comorbidity of Depression and Anxiety with Inflammatory Bowel Diseases. Inflamm. Bowel. Dis. 2016, 22, 752–762. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Pittet, V.; Rossel, J.-B.; von Känel, R. Swiss IBD Cohort Study Group Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 829–835.e1. [Google Scholar] [CrossRef]
- Mouzas, I.A.; Pallis, A.G.; Kochiadakis, G.E.; Marketou, M.; Chlouverakis, G.I.; Mellisas, J.; Vardas, P.E.; Kouroumalis, E.A. Autonomic Imbalance during the Day in Patients with Inflammatory Bowel Disease in Remission. Evidence from Spectral Analysis of Heart Rate Variability over 24 Hours. Dig. Liver. Dis. 2002, 34, 775–780. [Google Scholar] [CrossRef]
- Ganguli, S.C.; Kamath, M.V.; Redmond, K.; Chen, Y.; Irvine, E.J.; Collins, S.M.; Tougas, G. A Comparison of Autonomic Function in Patients with Inflammatory Bowel Disease and in Healthy Controls. Neurogastroenterol. Motil. 2007, 19, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Zawadka-Kunikowska, M.; Słomko, J.; Kłopocka, M.; Liebert, A.; Tafil-Klawe, M.; Klawe, J.J.; Newton, J.L.; Zalewski, P. Cardiac and Autonomic Function in Patients with Crohn’s Disease during Remission. Adv. Med. Sci. 2018, 63, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Nardi, D.; Zhang, M.; Yang, H.; Ombrellino, M.; Tracey, K.J. Role of Vagus Nerve Signaling in CNI-1493-Mediated Suppression of Acute Inflammation. Auton. Neurosci. 2000, 85, 141–147. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, H.; Chowdhury, R.; Abdi, T.; Selaru, F.; Chen, J.D.Z. Potential of Electrical Neuromodulation for Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2020, 26, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Picq, C.; Sinniger, V.; Mayol, J.F.; Clarençon, D. Vagus Nerve Stimulation: From Epilepsy to the Cholinergic Anti-Inflammatory Pathway. Neurogastroenterol. Motil. 2013, 25, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Attenello, F.; Amar, A.P.; Liu, C.; Apuzzo, M.L.J. Theoretical Basis of Vagus Nerve Stimulation. Prog. Neurol. Surg. 2015, 29, 20–28. [Google Scholar] [CrossRef]
- Payne, S.C.; Furness, J.B.; Burns, O.; Sedo, A.; Hyakumura, T.; Shepherd, R.K.; Fallon, J.B. Anti-Inflammatory Effects of Abdominal Vagus Nerve Stimulation on Experimental Intestinal Inflammation. Front. Neurosci. 2019, 13, 418. [Google Scholar] [CrossRef]
- Sinniger, V.; Pellissier, S.; Fauvelle, F.; Trocmé, C.; Hoffmann, D.; Vercueil, L.; Cracowski, J.-L.; David, O.; Bonaz, B. A 12-Month Pilot Study Outcomes of Vagus Nerve Stimulation in Crohn’s Disease. Neurogastroenterol. Motil. 2020, 32, e13911. [Google Scholar] [CrossRef]
- Révész, D.; Fröjd, V.; Rydenhag, B.; Ben-Menachem, E. Estimating Long-Term Vagus Nerve Stimulation Effectiveness: Accounting for Antiepileptic Drug Treatment Changes. Neuromodulation 2018, 21, 797–804. [Google Scholar] [CrossRef]
- Schreiber, S.; Reinisch, W.; Colombel, J.F.; Sandborn, W.J.; Hommes, D.W.; Robinson, A.M.; Huang, B.; Lomax, K.G.; Pollack, P.F. Subgroup Analysis of the Placebo-Controlled CHARM Trial: Increased Remission Rates through 3 Years for Adalimumab-Treated Patients with Early Crohn’s Disease. J. Crohns. Colitis 2013, 7, 213–221. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Cabrijan, Z.; Eberhardson, M.; van den Berg, R.M.; Löwenberg, M.; Danese, S.; Fiorino, G.; Levine, Y.A.; Chernof, D.N. 367–Vagus Nerve Stimulation Reduces Disease Activity and Modulates Serum and Autonomic Biomarkers in Biologicrefractory Crohn’s Disease. Gastroenterology 2019, 156, S-75. [Google Scholar] [CrossRef]
- Kibleur, A.; Pellissier, S.; Sinniger, V.; Robert, J.; Gronlier, E.; Clarençon, D.; Vercueil, L.; Hoffmann, D.; Bonaz, B.; David, O. Electroencephalographic Correlates of Low-Frequency Vagus Nerve Stimulation Therapy for Crohn’s Disease. Clin. Neurophysiol. 2018, 129, 1041–1046. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornaro, R.; Actis, G.C.; Caviglia, G.P.; Pitoni, D.; Ribaldone, D.G. Inflammatory Bowel Disease: Role of Vagus Nerve Stimulation. J. Clin. Med. 2022, 11, 5690. https://doi.org/10.3390/jcm11195690
Fornaro R, Actis GC, Caviglia GP, Pitoni D, Ribaldone DG. Inflammatory Bowel Disease: Role of Vagus Nerve Stimulation. Journal of Clinical Medicine. 2022; 11(19):5690. https://doi.org/10.3390/jcm11195690
Chicago/Turabian StyleFornaro, Riccardo, Giovanni Clemente Actis, Gian Paolo Caviglia, Demis Pitoni, and Davide Giuseppe Ribaldone. 2022. "Inflammatory Bowel Disease: Role of Vagus Nerve Stimulation" Journal of Clinical Medicine 11, no. 19: 5690. https://doi.org/10.3390/jcm11195690
APA StyleFornaro, R., Actis, G. C., Caviglia, G. P., Pitoni, D., & Ribaldone, D. G. (2022). Inflammatory Bowel Disease: Role of Vagus Nerve Stimulation. Journal of Clinical Medicine, 11(19), 5690. https://doi.org/10.3390/jcm11195690








