Surgical Treatment and Complications of Deep-Seated Nodular Plexiform Neurofibromas Associated with Neurofibromatosis Type 1
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Diagnosis
2.3. Surgical Procedure
2.4. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Surgical Outcomes
3.3. Factors Associated with Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woodruff, J.M. Pathology of tumors of the peripheral nerve sheath in type 1 neurofibromatosis. Am. J. Med. Genet. 1999, 89, 23–30. [Google Scholar] [CrossRef]
- Nguyen, R.; Dombi, E.; Widemann, B.C.; Solomon, J.; Fuensterer, C.; Kluwe, L.; Friedman, J.M.; Mautner, V.-F. Growth dynamics of plexiform neurofibromas: A retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J. Rare Dis. 2012, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, D.J.; Towbin, J.; Gottesman, G.; Gutmann, D.H. Clinic-based study of plexiform neurofibromas in neurofibromatosis 1. Am. J. Med. Genet. 2000, 92, 132–135. [Google Scholar] [CrossRef]
- Dombi, E.; Baldwin, A.; Marcus, L.J.; Fisher, M.J.; Weiss, B.; Kim, A.; Whitcomb, P.; Martin, S.; Aschbacher-Smith, L.E.; Rizvi, T.A.; et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N. Engl. J. Med. 2016, 375, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; Gutmann, D.H. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002, 62, 1573–1577. [Google Scholar]
- Korf, B.R. Plexiform neurofibromas. Am. J. Med. Genet. 1999, 89, 31–37. [Google Scholar] [CrossRef]
- Cnossen, M.H.; De Goede-Bolder, A.; Van Den Broek, K.M.; Waasdorp, C.M.E.; Oranje, A.P.; Stroink, H.; Simonsz, H.J.; van den Ouweland, A.M.; Halley, D.J.; Niermeijer, M.F. A prospective 10 year follow up study of patients with neurofibromatosis type 1. Arch. Dis. Child. 1998, 78, 408–412. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Aylsworth, A.; Carey, J.C.; Korf, B.; Marks, J.; Pyeritz, R.E.; Rubenstein, A.; Viskochil, D. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA 1997, 278, 51–57. [Google Scholar] [CrossRef]
- Yoshinaga, A.; Tsuge, I.; Yoshinaga, D.; Ogino, S.; Sakamoto, M.; Saito, S.; Morimoto, N. Reduction of intraoperative bleeding in diffuse plexiform neurofibroma resection using the ligaSure vessel sealing system. Plast Reconstr. Surg. 2021, 148, 344e–346e. [Google Scholar] [CrossRef]
- Konno, E.; Kishi, K. Use of the LigaSureTM vessel sealing system in neurofibroma excision to control postoperative bleeding. J. Plast. Reconstr. Aesthetic. Surg. 2012, 65, 814–817. [Google Scholar] [CrossRef]
- Michimoto, K.; Ashida, H.; Higuchi, T.; Kano, R.; Hasumi, J.; Suzuki, T.; Ishida, K.; Hirayama, H.; Ohta, A. Hemorrhagic complication in surgical resection for massive plexiform neurofibroma in body trunk: The flow-void sign as a predictor and preoperative embolization as prevention. World J. Surg. 2021, 45, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Canavese, F.; Krajbich, J.I. Resection of plexiform neurofibromas in children with neurofibromatosis type 1. J. Pediatr. Orthop. 2011, 31, 303–311. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Denadai, R.; Chang, C.-S.; Yao, C.-F.; Chen, Y.-A.; Chou, P.-Y.; Lo, L.-J.; Chen, Y.-R. The Number of Surgical Interventions and Specialists Involved in the Management of Patients with Neurofibromatosis Type I: A 25-Year Analysis. J. Pers. Med. 2022, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Donner, T.R.; Voorhies, R.M.; Kline, D.G. Neural sheath tumors of major nerves. J. Neurosurg. 1994, 81, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Tydings, C.; Yarmolenko, P.; Bornhorst, M.; Dombi, E.; Myseros, J.; Keating, R.; Bost, J.; Sharma, K.; Kim, A. Feasibility of magnetic resonance-guided high-intensity focused ultrasound treatment targeting distinct nodular lesions in neurofibromatosis type 1. Neurooncol. Adv. 2021, 3, vdab116. [Google Scholar] [CrossRef]
- Akshintala, S.; Baldwin, A.; Liewehr, D.J.; Goodwin, A.; Blakeley, J.O.; Gross, A.M.; Steinberg, S.M.; Dombi, E.; Widemann, B.C. Longitudinal evaluation of peripheral nerve sheath tumors in neurofibromatosis type 1: Growth analysis of plexiform neurofibromas and distinct nodular lesions. Neuro Oncol. 2020, 22, 1368–1378. [Google Scholar] [CrossRef]
- Nishida, Y.; Ikuta, K.; Natsume, A.; Ishihara, N.; Morikawa, M.; Kidokoro, H.; Muramatsu, Y.; Nonobe, N.; Ishizuka, K.; Takeichi, T.; et al. Establishment of in-hospital clinical network for patients with neurofibromatosis type 1 in Nagoya University Hospital. Sci. Rep. 2021, 11, 11933. [Google Scholar] [CrossRef]
- Wasa, J.; Nishida, Y.; Tsukushi, S.; Shido, Y.; Sugiura, H.; Nakashima, H.; Ishiguro, N. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am. J. Roentgenol. 2010, 194, 1568–1574. [Google Scholar] [CrossRef]
- Koike, H.; Nishida, Y.; Ito, S.; Shimoyama, Y.; Ikuta, K.; Urakawa, H.; Sakai, T.; Shimizu, K.; Ito, K.; Imagama, S. Diffusion-weighted magnetic resonance imaging improves the accuracy of differentiation of benign from malignant peripheral nerve sheath tumors. World Neurosurg. 2022, 157, e207–e214. [Google Scholar] [CrossRef]
- Nishida, Y.; Ikuta, K.; Ito, S.; Urakawa, H.; Sakai, T.; Koike, H.; Ito, K.; Imagama, S. Limitations and benefits of FDG-PET/CT in NF1 patients with nerve sheath tumors: A cross-sectional/longitudinal study. Cancer Sci. 2021, 112, 1114–1122. [Google Scholar] [CrossRef]
- Miettinen, M.M.; Antonescu, C.R.; Fletcher, C.D.M.; Kim, A.; Lazar, A.J.; Quezado, M.M.; Reilly, K.M.; Stemmer-Rachamimov, A.; Stewart, D.R.; Viskochil, D.; et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum. Pathol. 2017, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Murovic, J.A.; Tiel, R.L.; Kline, D.G. Operative outcomes of 546 Louisiana State University Health Sciences Center peripheral nerve tumors. Neurosurg. Clin. N. Am. 2004, 15, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Ibrahim, C.; Friedrich, R.E.; Westphal, M.; Schuhmann, M.; Mautner, V.F. Growth behavior of plexiform neurofibromas after surgery. Genet. Med. 2013, 15, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Prada, C.E.; Rangwala, F.A.; Martin, L.J.; Lovell, A.M.; Saal, H.M.; Schorry, E.K.; Hopkin, R.J. Pediatric plexiform neurofibromas: Impact on morbidity and mortality in neurofibromatosis type 1. J. Pediatr. 2012, 160, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Staser, K.; Yang, F.-C.; Clapp, D.W. Pathogenesis of plexiform neurofibroma: Tumor-stromal/hematopoietic interactions in tumor progression. Annu. Rev. Pathol. 2012, 7, 469–495. [Google Scholar] [CrossRef]
- Nelson, C.N.; Dombi, E.; Rosenblum, J.S.; Miettinen, M.M.; Lehky, T.J.; Whitcomb, P.O.; Hayes, C.; Scott, G.; Benzo, S.; Widemann, B.C.; et al. Safe marginal resection of atypical neurofibromas in neurofibromatosis type 1. J. Neurosurg. 2019, 133, 1516–1526. [Google Scholar] [CrossRef]
- Needle, M.N.; Cnaan, A.; Dattilo, J.; Chatten, J.; Phillips, P.C.; Shochat, S.; Sutton, L.N.; Vaughan, S.N.; Zackai, E.H.; Zhao, H.; et al. Prognostic signs in the surgical management of plexiform neurofibroma: The Children’s Hospital of Philadelphia experience, 1974–1994. J. Pediatr. 1997, 131, 678–682. [Google Scholar] [CrossRef]
- Kim, D.H.; Murovic, J.A.; Tiel, R.L.; Moes, G.; Kline, D.G. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J. Neurosurg. 2005, 102, 246–255. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, J.Y.; Lee, Y.S.; Jeong, H.S. Intramuscular peripheral nerve sheath tumors: Schwannoma, ancient schwannoma, and neurofibroma. Skeletal Radiol. 2020, 49, 967–975. [Google Scholar] [CrossRef]
- Kehoe, N.J.S.; Reid, R.P.; Semple, J.C. Solitary benign peripheral-nerve tumor: Review of 32 years’ experience. J. Bone Jt. Surg. Br. 1995, 77, 497–500. [Google Scholar] [CrossRef]
- Perez-Roman, R.J.; Shelby Burks, S.; Debs, L.; Cajigas, I.; Levi, A.D. The risk of peripheral nerve tumor biopsy in suspected benign etiologies. Neurosurgery 2020, 86, E326–E332. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Desai, K.; Agrawal, N.; Mirchandani, K.; Chatterjee, S.; Sarpong, E.; Sen, S. Characteristics, treatment patterns, healthcare resource use, and costs among pediatric patients diagnosed with neurofibromatosis type 1 and plexiform neurofibromas: A retrospective database analysis of a medicaid population. Curr. Med. Res. Opin. 2021, 37, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Singh, G.; Akshintala, S.; Baldwin, A.; Dombi, E.; Ukwuani, S.; Goodwin, A.; Liewehr, D.J.; Steinberg, S.M.; Widemann, B.C. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018, 20, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
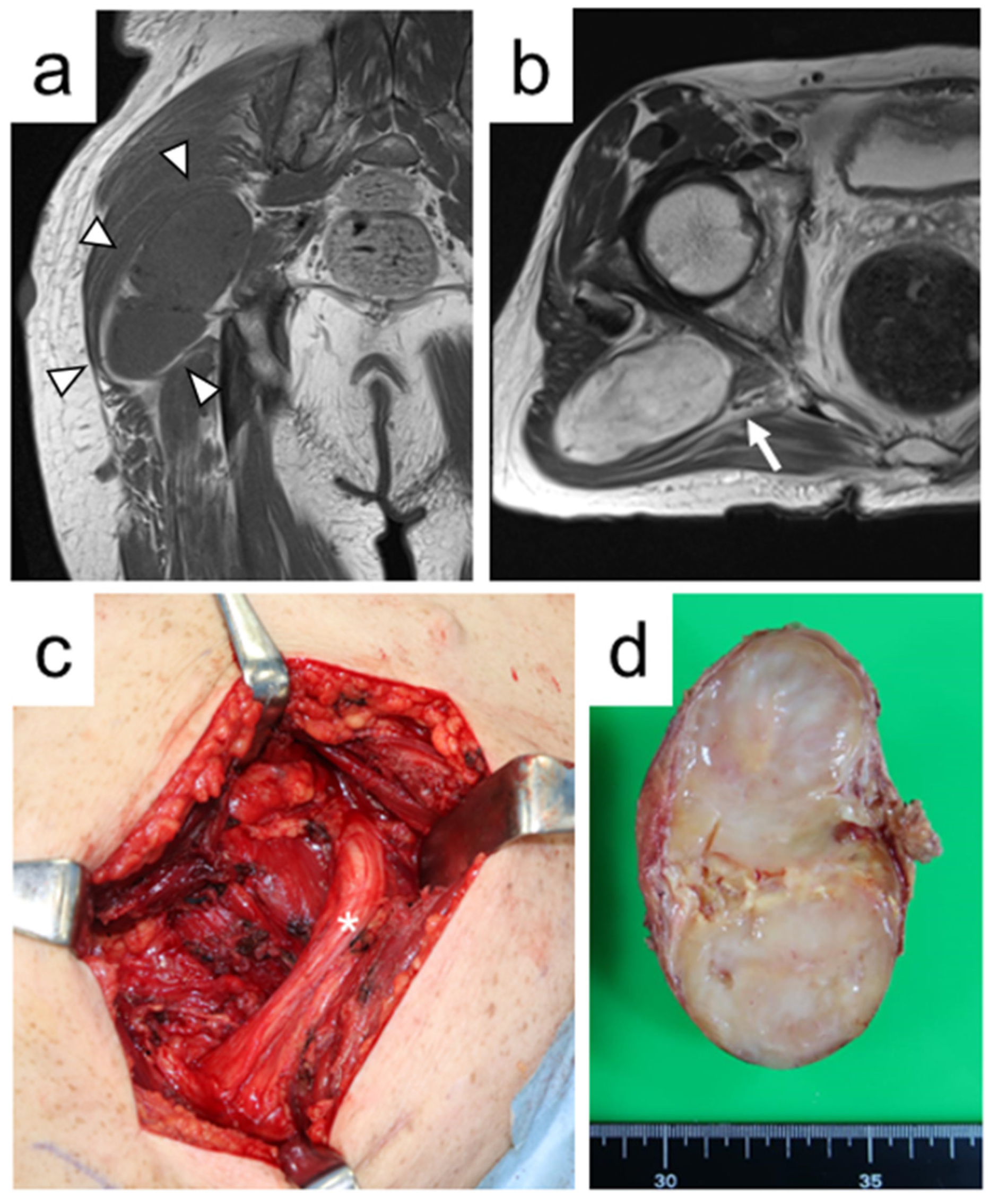
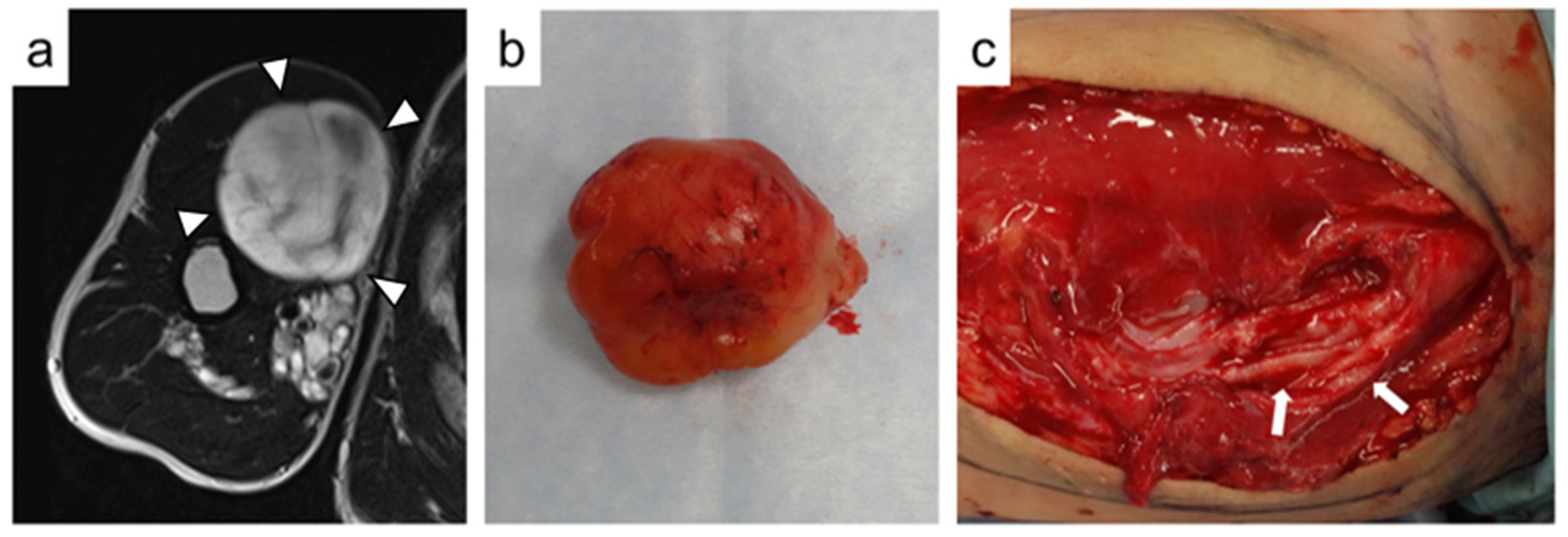
| Case No. | Age at Surgery (ys) | Gender | Number of Tumors | Indication for Surgery | Duration of Symptoms (ms) | Follow-Up Duration (ms) |
|---|---|---|---|---|---|---|
| 1 | 38 | F | 1 | numbness | 26 | 44 |
| 2 | 32 | F | 1 | pain | 6 | 42 |
| 3 | 43 | M | 1 | tumor growing | - | 46 |
| 4 | 22 | M | 1 | muscle weakness | 3 | 69 |
| 5 | 29 | F | 1 | pain | 5 | 67 |
| 6 | 28 | F | 4 | tumor growing | - | 14 |
| 7 | 55 | M | 1 | pain | 2 | 43 |
| 8 | 26 | M | 1 | pain | 18 | 68 |
| 9 | 14 | M | 1 | tumor growing | 12 | 72 |
| 10 | 17 | M | 1 | tumor growing | - | 41 |
| 11 | 14 | F | 2 | pain | 8 | 52 |
| 12 | 39 | F | 6 | pain | 4 | 60 |
| 13 | 17 | F | 1 | pain | 19 | 51 |
| 14 | 12 | M | 1 | pain | 13 | 88 |
| 15 | 36 | F | 1 | tumor growing | - | 44 |
| Tumor No. | Site | Location | Size (cm) | Surgical Methods | Postoperative Complications |
|---|---|---|---|---|---|
| 1 | back | intramuscular | 6.1 | en bloc resection | - |
| 2 | back | intramuscular | 8.7 | en bloc resection | - |
| 3 | back | intramuscular | 4.0 | en bloc resection | - |
| 4 | lower leg | intermuscular (tibial nerve) | 5.0 | enucleation | - |
| 5 | buttock | intermuscular (sciatic nerve) | 5.8 | enucleation | - |
| 6 | buttock | intermuscular | 5.6 | enucleation | - |
| 7 | buttock | intramuscular | 5.0 | enucleation | - |
| 8 | thigh | intermuscular (sciatic nerve) | 5.8 | enucleation | - |
| 9 | thigh | intramuscular | 2.6 | enucleation | - |
| 10 | buttock | intermuscular | 6.8 | en bloc resection | - |
| 11 | upper arm | intermuscular (musculocutaneous nerve) | 8.0 | enucleation | bleeding |
| 12 | lower leg | intramuscular | 6.5 | en bloc resection | sensory deficits (transient) |
| 13 | retroperitoneum | intermuscular (femoral nerve) | 6.6 | enucleation | muscle weakness (transient) |
| 14 | upper arm | intramuscular | 1.9 | enucleation | - |
| 15 | neck | intramuscular | 0.8 | enucleation | - |
| 16 | back | intramuscular | 11.4 | enucleation | - |
| 17 | thigh | intermuscular | 5.1 | enucleation | - |
| 18 | thigh | intramuscular | 6.5 | enucleation | - |
| 19 | thigh | intramuscular | 7.3 | enucleation | - |
| 20 | thigh | intramuscular | 5.0 | enucleation | - |
| 21 | thigh | intermuscular (saphenous nerve) | 4.3 | enucleation | sensory deficits (persistent) |
| 22 | buttock | intramuscular | 4.3 | en bloc resection | - |
| 23 | lower leg | intramuscular | 10.6 | en bloc resection | - |
| 24 | retroperitoneum | intermuscular (femoral nerve) | 6.8 | en bloc resection | muscle weakness and sensory deficits (persistent) |
| Variables | Number of Tumors (n = 24) | Tumors without Neurological Complications (n = 20) | Tumors with Neurological Complications (n = 4) | p Value |
|---|---|---|---|---|
| Tumor size a (median, cm) | 5.8 | 5.6 | 6.6 | 0.63 |
| Tumor location | 0.27 | |||
| Intramuscular | 14 | 13 | 1 | |
| Others | 10 | 7 | 3 | |
| Presence of biopsy | 1.0 | |||
| Yes | 5 | 4 | 1 | |
| No | 19 | 16 | 3 | |
| Surgical methods | 0.58 | |||
| Enucleation | 16 | 14 | 2 | |
| En bloc resection | 8 | 6 | 2 | |
| Types of parent nerve | 0.25 | |||
| Major nerve | 6 | 4 | 2 | |
| Minor nerve | 18 | 16 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuta, K.; Nishida, Y.; Sakai, T.; Koike, H.; Ito, K.; Urakawa, H.; Imagama, S. Surgical Treatment and Complications of Deep-Seated Nodular Plexiform Neurofibromas Associated with Neurofibromatosis Type 1. J. Clin. Med. 2022, 11, 5695. https://doi.org/10.3390/jcm11195695
Ikuta K, Nishida Y, Sakai T, Koike H, Ito K, Urakawa H, Imagama S. Surgical Treatment and Complications of Deep-Seated Nodular Plexiform Neurofibromas Associated with Neurofibromatosis Type 1. Journal of Clinical Medicine. 2022; 11(19):5695. https://doi.org/10.3390/jcm11195695
Chicago/Turabian StyleIkuta, Kunihiro, Yoshihiro Nishida, Tomohisa Sakai, Hiroshi Koike, Kan Ito, Hiroshi Urakawa, and Shiro Imagama. 2022. "Surgical Treatment and Complications of Deep-Seated Nodular Plexiform Neurofibromas Associated with Neurofibromatosis Type 1" Journal of Clinical Medicine 11, no. 19: 5695. https://doi.org/10.3390/jcm11195695






