Biomarkers for the Prediction and Judgement of Sepsis and Sepsis Complications: A Step towards precision medicine?
Abstract
:1. Introduction
2. What Are Biomarkers?
3. The Role of Biomarkers in precision medicine
4. Will Biomarkers Pave the Way for precision medicine Sepsis Trials?
5. Three Exemplary Use Cases for Biomarkers in the Context of Sepsis
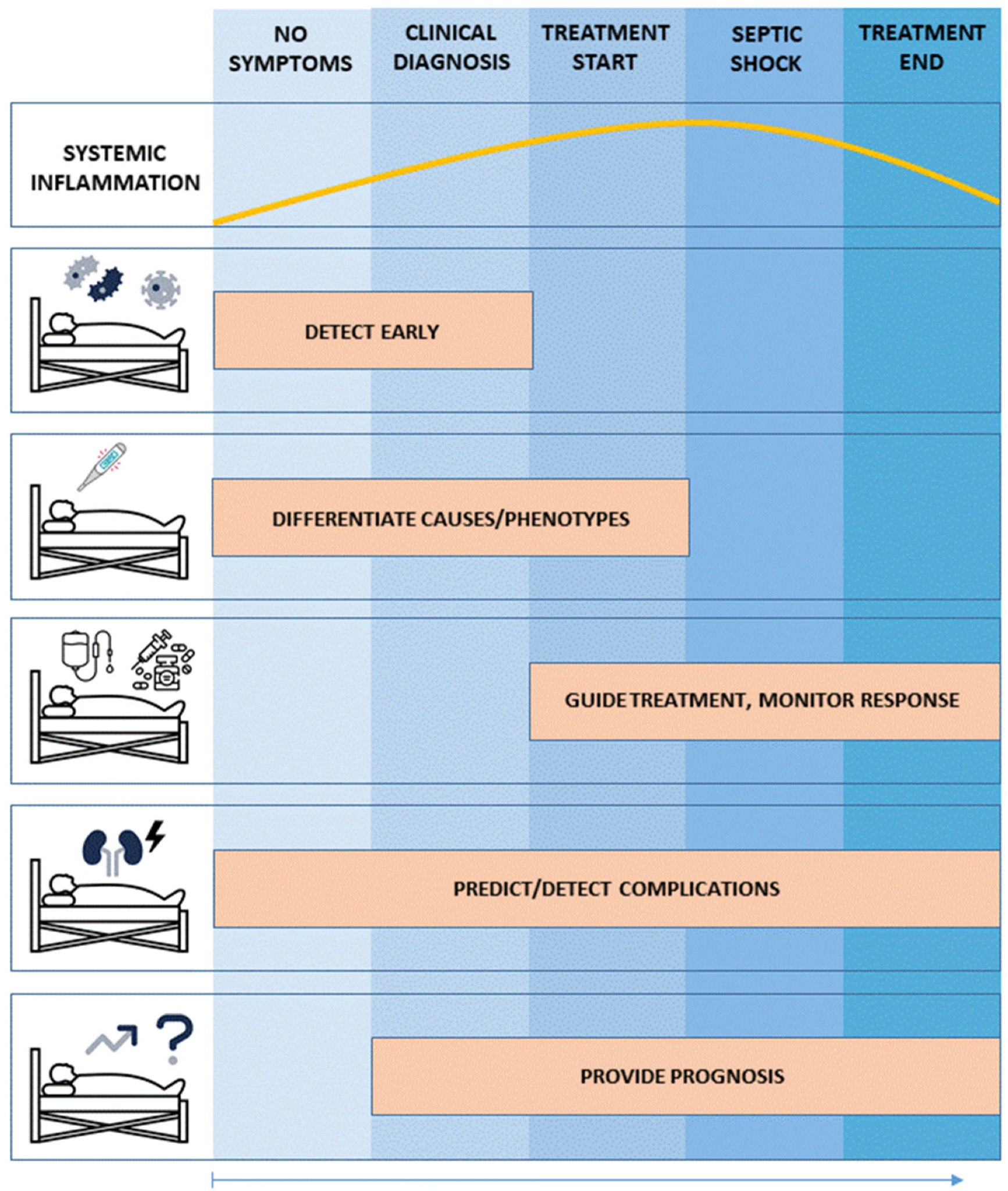
5.1. Use Case No. 1: Biomarker-Guided Evaluation and Therapy of Patients with Dysregulated Systemic Inflammation and Sepsis: Implementing a precision medicine Approach
5.2. Use Case No. 2: Specific Biomarkers for Specific Therapies
5.3. Use Case No. 3: Biomarker-Guided precision medicine to Treat Sepsis Complications: Sepsis-Associated Acute Kidney Injury (sAKI)
6. Outlook: The Coming Era of—Omics Technology?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of global incidence and mortality of hospital-treated sepsis—Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.R.; Payen, D. Sepsis in European intensive care units: Results of the SOAP study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Lagu, T.; Rothberg, M.B.; Shieh, M.S.; Pekow, P.S.; Steingrub, J.S.; Lindenauer, P.K. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit. Care Med. 2012, 40, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Harrison, D.A.; Rubenfeld, G.D.; Rowan, K. Epidemiology of sepsis and septic shock in critical care units: Comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br. J. Anaesth. 2017, 119, 626–636. [Google Scholar] [CrossRef]
- White, L.E.; Hassoun, H.T.; Bihorac, A.; Moore, L.J.; Sailors, R.M.; McKinley, B.A.; Valdivia, A.; Moore, F.A. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J. Trauma Acute Care Surg. 2013, 75, 432–438. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Wong, H.R. Intensive care medicine in 2050: Precision medicine. Intensive Care Med. 2017, 43, 1507–1509. [Google Scholar] [CrossRef] [Green Version]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Agusti, A.; Bel, E.; Thomas, M.; Vogelmeier, C.; Brusselle, G.; Holgate, S.; Humbert, M.; Jones, P.; Gibson, P.G.; Vestbo, J.; et al. Treatable traits: Toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016, 47, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Precision medicine needs an equity agenda. Nat. Med. 2021, 27, 737. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Carlson, J.J.; Veenstra, D.L. A Framework for Prioritizing Research Investments in Precision Medicine. Med. Decis. Mak. 2016, 36, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.H.; Giacomini, K.M. Oral Anticoagulants and Precision Medicine: Something Old, Something New. Clin. Pharmacol. Ther. 2020, 107, 1273–1277. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Lew, D. Reconciling evidence-based medicine and precision medicine in the era of big data: Challenges and opportunities. Genome Med. 2016, 8, 134. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharm. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Reinhart, K. International Sepsis Forum. Biomarkers of sepsis. Crit. Care Med. 2009, 37, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R. Sepsis Biomarkers. J. Pediatr. Intensive Care 2019, 8, 11–16. [Google Scholar] [CrossRef]
- Vincent, J.L. We should abandon randomized controlled trials in the intensive care unit. Crit. Care Med. 2010, 38 (Suppl. 10), S534–S538. [Google Scholar] [CrossRef]
- Ospina-Tascón, G.A.; Büchele, G.L.; Vincent, J.L. Multicenter, randomized, controlled trials evaluating mortality in intensive care: Doomed to fail? Crit. Care Med. 2008, 36, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I. Negative Trials, and What to Do with Them?: First, Stop Calling Them “Negative”. Anesthesiology 2020, 132, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Laffey, J.G.; Kavanagh, B.P. Negative trials in critical care: Why most research is probably wrong. Lancet Respir. Med. 2018, 6, 659–660. [Google Scholar] [CrossRef]
- Chaudhary, K.; Vaid, A.; Duffy, Á.; Paranjpe, I.; Jaladanki, S.; Paranjpe, M.; Johnson, K.; Gokhale, A.; Pattharanitima, P.; Chauhan, K.; et al. Utilization of Deep Learning for Subphenotype Identification in Sepsis-Associated Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. CJASN 2020, 15, 1557–1565. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Wong, H.R.; Atkinson, S.J.; Cvijanovich, N.Z.; Anas, N.; Allen, G.L.; Thomas, N.J.; Bigham, M.T.; Weiss, S.L.; Fitzgerald, J.C.; Checchia, P.A.; et al. Combining Prognostic and Predictive Enrichment Strategies to Identify Children With Septic Shock Responsive to Corticosteroids. Crit. Care Med. 2016, 44, e1000–e1003. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.J.; Tsalik, E.L.; van Velkinburgh, J.C.; Glickman, S.W.; Rice, B.J.; Wang, C.; Chen, B.; Carin, L.; Suarez, A.; Mohney, R.P.; et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 2013, 5, 195ra95. [Google Scholar] [CrossRef]
- Meyer, N.J.; Feng, R.; Li, M.; Zhao, Y.; Sheu, C.C.; Tejera, P.; Gallop, R.; Bellamy, S.; Rushefski, M.; Lanken, P.N.; et al. IL1RN coding variant is associated with lower risk of acute respiratory distress syndrome and increased plasma IL-1 receptor antagonist. Am. J. Respir. Crit. Care Med. 2013, 187, 950–959. [Google Scholar] [CrossRef]
- Meyer, N.J.; Ferguson, J.F.; Feng, R.; Wang, F.; Patel, P.N.; Li, M.; Xue, C.; Qu, L.; Liu, Y.; Boyd, J.H.; et al. A functional synonymous coding variant in the IL1RN gene is associated with survival in septic shock. Am. J. Respir. Crit. Care Med. 2014, 190, 656–664. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Peters van Ton, A.M.; Kox, M.; Abdo, W.F.; Pickkers, P. Precision Immunotherapy for Sepsis. Front. Immunol. 2018, 9, 1926. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.P.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; Martin, C.; et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N. Engl. J. Med. 2018, 378, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.J.; Fisher, C.J., Jr.; Sprung, C.L.; Straube, R.C.; Sadoff, J.C.; Foulke, G.E.; Wortel, C.H.; Fink, M.P.; Dellinger, R.P.; Teng, N.N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N. Engl. J. Med. 1991, 324, 429–436. [Google Scholar] [CrossRef]
- Fisher, C.J., Jr.; Slotman, G.J.; Opal, S.M.; Pribble, J.P.; Bone, R.C.; Emmanuel, G.; Ng, D.; Bloedow, D.C.; Catalano, M.A.; IL-1RA Sepsis Syndrome Study Group. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: A randomized, open-label, placebo-controlled multicenter trial. Crit. Care Med. 1994, 22, 12–21. [Google Scholar] [CrossRef]
- Opal, S.M.; Dellinger, R.P.; Vincent, J.L.; Masur, H.; Angus, D.C. The next generation of sepsis clinical trial designs: What is next after the demise of recombinant human activated protein C?*. Crit. Care Med. 2014, 42, 1714–1721. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Hofer, N.; Zacharias, E.; Müller, W.; Resch, B. An Update on the Use of C-Reactive Protein in Early-Onset Neonatal Sepsis: Current Insights and New Tasks. Neonatology 2012, 102, 25–36. [Google Scholar] [CrossRef]
- Ryu, J.-A.; Yang, J.H.; Lee, D.; Park, C.-M.; Suh, G.Y.; Jeon, K.; Cho, J.; Baek, S.Y.; Carriere, K.C.; Chung, C.R. Clinical Usefulness of Procalcitonin and C-Reactive Protein as Outcome Predictors in Critically Ill Patients with Severe Sepsis and Septic Shock. PLoS ONE 2015, 10, e0138150. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, A.L.; Vanimaya; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; Manoj, G. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.M.; Eslick, G.D.; Craig, J.C.; McLean, A.S. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: Systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 210–217. [Google Scholar] [CrossRef]
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef]
- de Jong, E.; A van Oers, J.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; E Haas, L.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: A randomised, controlled, open-label trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Liaskou-Antoniou, L.; Adamis, G.; Panagaki, A.; Melachroinopoulos, N.; Drakou, E.; Marousis, K.; Chrysos, G.; Spyrou, A.; Alexiou, N.; et al. Procalcitonin to Reduce Long-Term Infection-associated Adverse Events in Sepsis. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 202–210. [Google Scholar] [CrossRef]
- Wirz, Y.; Meier, M.A.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Schroeder, S.; Nobre, V.; Annane, D.; et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit. Care 2018, 22, 191. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Rhee, C.; Welsh, J.; Powers, J.H.; Danner, R.L.; Kadri, S.S. Procalcitonin-Guided Antibiotic Discontinuation and Mortality in Critically Ill Adults. Chest 2019, 155, 1109–1118. [Google Scholar] [CrossRef]
- A Amer, H.; Ghareeb, H.; Lotfy, N.M.; O El-Azizi, N.; Mahmoud, A.M. Presepsin a Diagnostic Marker for Sepsis in Intensive Care Unit Patients. Egypt. J. Immunol. 2016, 23, 109–118. [Google Scholar]
- Masson, S.; Caironi, P.; Fanizza, C.; Thomae, R.; Bernasconi, R.; Noto, A.; Oggioni, R.; Pasetti, G.S.; Romero, M.; Tognoni, G.; et al. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: Data from the multicenter, randomized ALBIOS trial. Intensive Care Med. 2014, 41, 12–20. [Google Scholar] [CrossRef]
- Leli, C.; Ferranti, M.; Marrano, U.; Al Dhahab, Z.S.; Bozza, S.; Cenci, E.; Mencacci, A. Diagnostic accuracy of presepsin (sCD14-ST) and procalcitonin for prediction of bacteraemia and bacterial DNAaemia in patients with suspected sepsis. J. Med. Microbiol. 2016, 65, 713–719. [Google Scholar] [CrossRef]
- Kim, H.; Hur, M.; Moon, H.-W.; Yun, Y.-M.; Di Somma, S.; Network, G. Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann. Intensive Care 2017, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, G.; Shibata, S.; Fukui, Y.; Okamura, Y.; Inoue, Y. Diagnostic accuracy of procalcitonin and presepsin for infectious disease in patients with acute kidney injury. Diagn. Microbiol. Infect. Dis. 2016, 86, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Magudumana, O.; Ballot, D.; Cooper, P.; Trusler, J.; Cory, B.; Viljoen, E.; Carter, A. Serial interleukin 6 measurements in the early diagnosis of neonatal sepsis. J. Trop. Pediatr. 2000, 46, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, C. Diagnostic value of a combination of biomarkers in patients with sepsis and severe sepsis in emergency department. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014, 26, 153–158. [Google Scholar] [CrossRef]
- Henning, D.J.; Hall, M.K.; Watsjold, B.K.; Bhatraju, P.K.; Kosamo, S.; Shapiro, N.I.; Liles, W.C.; Wurfel, M.M. Interleukin-6 improves infection identification when added to physician judgment during evaluation of potentially septic patients. Am. J. Emerg. Med. 2020, 38, 947–952. [Google Scholar] [CrossRef]
- Mat-Nor, M.B.; Ralib, A.M.; Abdulah, N.Z.; Pickering, J.W. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. J. Crit. Care 2016, 33, 245–251. [Google Scholar] [CrossRef]
- Stryjewski, G.R.; Nylen, E.S.; Bell, M.J.; Snider, R.H.; Becker, K.L.; Wu, A.; Lawlor, C.; Dalton, H. Interleukin-6, interleukin-8, and a rapid and sensitive assay for calcitonin precursors for the determination of bacterial sepsis in febrile neutropenic children. Pediatr. Crit. Care Med. 2005, 6, 129–135. [Google Scholar] [CrossRef]
- Dimoula, A.; Pradier, O.; Kassengera, Z.; Dalcomune, D.; Turkan, H.; Vincent, J.-L. Serial Determinations of Neutrophil CD64 Expression for the Diagnosis and Monitoring of Sepsis in Critically Ill Patients. Clin. Infect. Dis. 2013, 58, 820–829. [Google Scholar] [CrossRef]
- Soni, S.; Wadhwa, N.; Kumar, R.; Faridi, M.; Sharma, S.; Chopra, A.; Singh, S. Evaluation of CD64 Expression on Neutrophils as an Early Indicator of Neonatal Sepsis. Pediatr. Infect. Dis. J. 2013, 32, e33–e37. [Google Scholar] [CrossRef]
- Cardelli, P.; Ferraironi, M.; Amodeo, R.; Tabacco, F.; De Blasi, R.; Nicoletti, M.; Sessa, R.; Petrucca, A.; Costante, A.; Cipriani, P. Evaluation of Neutrophil CD64 Expression and Procalcitonin as Useful Markers in Early Diagnosis of Sepsis. Int. J. Immunopathol. Pharmacol. 2008, 21, 43–49. [Google Scholar] [CrossRef]
- Gámez-Díaz, L.Y.; Enriquez, L.E.; Matute, J.D.; Velásquez, S.; Gómez, I.D.; Toro, F.; Ospina, S.; Bedoya, V.; Arango, C.M.; Valencia, M.L.; et al. Diagnostic Accuracy of HMGB-1, sTREM-1, and CD64 as Markers of Sepsis in Patients Recently Admitted to the Emergency Department. Acad. Emerg. Med. 2011, 18, 807–815. [Google Scholar] [CrossRef]
- Rogina, P.; Stubljar, D.; Lejko-Zupanc, T.; Osredkar, J.; Skvarc, M. Expression of CD64 on neutrophils (CD64 index): Diagnostic accuracy of CD64 index to predict sepsis in critically ill patients. Clin. Chem. Lab. Med. (CCLM) 2015, 53, e89–e91. [Google Scholar] [CrossRef] [PubMed]
- Ponte, S.T.D.; Alegretti, A.P.; Pilger, D.A.; Rezende, G.P.; Andrioli, G.; Ludwig, H.C.; Diogo, L.; Goldani, L.Z.; Loreto, M.; Machado, P.S.; et al. Diagnostic Accuracy of CD64 for Sepsis in Emergency Department. J. Glob. Infect. Dis. 2018, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Jämsä, J.; Ala-Kokko, T.; Huotari, V.; Ohtonen, P.; Savolainen, E.-R.; Syrjälä, H. Neutrophil CD64, C-reactive protein, and procalcitonin in the identification of sepsis in the ICU—Post-test probabilities. J. Crit. Care 2018, 43, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H.E.; El Masry, S.A.; Mokhtar, A.M.; Ismail, E.A.; Abdelaal, N.M. Valuable Role of Neutrophil CD64 and Highly Sensitive CRP Biomarkers for Diagnostic, Monitoring, and Prognostic Evaluations of Sepsis Patients in Neonatal ICUs. BioMed Res. Int. 2020, 2020, 6214363. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, J.; Fei, A.; Wang, F.; Pan, S.; Wang, W. Neutrophil CD64 expression is a predictor of mortality for patients in the intensive care unit. Int. J. Clin. Exp. Pathol. 2014, 7, 7806–7813. [Google Scholar] [PubMed]
- Chaturvedi, S.; Mishra, P.; Mishra, R.; Ghosh, P.S.; Singh, H.; Baronia, A.K.; Singh, R.K. Correlation of neutrophil CD64 with clinical profile and outcome of sepsis patients during intensive care unit stay. Indian J. Crit. Care Med. 2018, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Stortz, J.A.; Murphy, T.J.; Raymond, S.L.; Mira, J.C.; Ungaro, R.; Dirain, M.L.; Nacionales, D.C.; Loftus, T.; Wang, Z.; Ozrazgat-Baslanti, T.; et al. Evidence for Persistent Immune Suppression in Patients Who Develop Chronic Critical Illness After Sepsis. Shock 2018, 49, 249–258. [Google Scholar] [CrossRef]
- Wang, J.-F.; Li, J.-B.; Zhao, Y.-J.; Yi, W.-J.; Bian, J.-J.; Wan, X.-J.; Zhu, K.-M.; Deng, X.-M. Up-regulation of Programmed Cell Death 1 Ligand 1 on Neutrophils May Be Involved in Sepsis-induced Immunosuppression. Anesthesiology 2015, 122, 852–863. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Colston, E.; Yende, S.; Angus, D.C.; Moldawer, L.L.; Crouser, E.D.; Martin, G.; Coopersmith, C.M.; Brakenridge, S.; Mayr, F.B.; et al. Immune Checkpoint Inhibition in Sepsis. Crit. Care Med. 2019, 47, 632–642. [Google Scholar] [CrossRef]
- Fernández-Grande, E.; Cabrera, C.M.; González, B.; Varela, C.; Urra, J.M. Enhanced HLA-DR expression on T-lymphocytes from patients in early stages of non-surgical sepsis. Med. Clínica 2018, 152, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Tschaikowsky, K.; Hedwig-Geissing, M.; Schiele, A.; Bremer, F.; Schywalsky, M.; Schüttler, J. Coincidence of pro- and anti-inflammatory responses in the early phase of severe sepsis: Longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit. Care Med. 2002, 30, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Lepape, A.; Voirin, N.; Bohé, J.; Venet, F.; Debard, A.-L.; Thizy, H.; Bienvenu, J.; Gueyffier, F.; Vanhems, P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006, 32, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Quadrini, K.J.; Patti-Diaz, L.; Maghsoudlou, J.; Cuomo, J.; Hedrick, M.N.; McCloskey, T.W. A flow cytometric assay for HLA-DR expression on monocytes validated as a biomarker for enrollment in sepsis clinical trials. Cytom. Part B Clin. Cytom. 2021, 100, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Caironi, P.; Masson, S.; Mauri, T.; Bottazzi, B.; Leone, R.; Magnoli, M.; Barlera, S.; Mamprin, F.; Fedele, A.; Mantovani, A.; et al. Pentraxin 3 in patients with severe sepsis or shock: The ALBIOS trial. Eur. J. Clin. Investig. 2016, 47, 73–83. [Google Scholar] [CrossRef]
- Mauri, T.; Bellani, G.; Patroniti, N.; Coppadoro, A.; Peri, G.; Cuccovillo, I.; Cugno, M.; Iapichino, G.; Gattinoni, L.; Pesenti, A.; et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010, 36, 621–629. [Google Scholar] [CrossRef]
- Song, J.; Moon, S.; Park, D.W.; Cho, H.-J.; Kim, J.Y.; Park, J.; Cha, J.H. Biomarker combination and SOFA score for the prediction of mortality in sepsis and septic shock. Medicine 2020, 99, e20495. [Google Scholar] [CrossRef]
- Schreiber, H.; Rittirsch, D.; Flierl, M.; Brueckner, U.; Schneider, M.; Weiss, M.; Gebhard, F.; Huber-Lang, M. Complement Activation During Sepsis in Humans. Curr. Top. Complement 2006, 586, 217–226. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Day, D.E.; Zetoune, F.S.; Sarma, J.V.; Huber-Lang, M.S.; Ward, P.A. Functions of the complement components C3 and C5 during sepsis. FASEB J. 2008, 22, 3483–3490. [Google Scholar] [CrossRef]
- Sakr, Y.; Burgett, U.; Nacul, F.E.; Reinhart, K.; Brunkhorst, F. Lipopolysaccharide binding protein in a surgical intensive care unit: A marker of sepsis?*. Crit. Care Med. 2008, 36, 2014–2022. [Google Scholar] [CrossRef]
- Kumar, S.; Ingle, H.; Prasad, D.V.R.; Kumar, H. Recognition of bacterial infection by innate immune sensors. Crit. Rev. Microbiol. 2012, 39, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.; Rottman, M.; Shapiro, N.I.; Seiler, B.; Lombardo, P.; Gamini, N.; Tomolonis, J.; Watters, A.L.; Waterhouse, A.; Leslie, D.; et al. A Broad-Spectrum Infection Diagnostic that Detects Pathogen-Associated Molecular Patterns (PAMPs) in Whole Blood. eBioMedicine 2016, 9, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, A.; Yuzawa, J.; Klein, D.J.; Takeda, M.; Harada, T. Combining intermediate levels of the Endotoxin Activity Assay (EAA) with other biomarkers in the assessment of patients with sepsis: Results of an observational study. Crit. Care 2012, 16, R88. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Kono, H.; Patel, Z.; Rock, K.L. Evaluation of the Contribution of Multiple DAMPs and DAMP Receptors in Cell Death-Induced Sterile Inflammatory Responses. PLoS ONE 2014, 9, e104741. [Google Scholar] [CrossRef]
- Piotti, A.; Novelli, D.; Meessen, J.M.T.A.; Ferlicca, D.; Coppolecchia, S.; Marino, A.; Salati, G.; Savioli, M.; Grasselli, G.; Bellani, G.; et al. Endothelial damage in septic shock patients as evidenced by circulating syndecan-1, sphingosine-1-phosphate and soluble VE-cadherin: A substudy of ALBIOS. Crit. Care 2021, 25, 113. [Google Scholar] [CrossRef]
- Johansen, M.E.; Johansson, P.I.; Ostrowski, S.R.; Bestle, M.H.; Hein, L.; Jensen, A.L.G.; Søe-Jensen, P.; Andersen, M.H.; Steensen, M.; Mohr, T.; et al. Profound Endothelial Damage Predicts Impending Organ Failure and Death in Sepsis. Semin. Thromb. Hemost. 2015, 41, 16–25. [Google Scholar] [CrossRef]
- Ikeda, M.; Matsumoto, H.; Ogura, H.; Hirose, T.; Shimizu, K.; Yamamoto, K.; Maruyama, I.; Shimazu, T. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J. Crit. Care 2018, 43, 48–53. [Google Scholar] [CrossRef]
- Ostrowski, S.R.; Haase, N.; Müller, R.B.; Møller, M.H.; Pott, F.C.; Perner, A.; Johansson, P.I. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: A prospective study. Crit. Care 2015, 19, 191. [Google Scholar] [CrossRef]
- Straat, M.; Müller, M.C.; Meijers, J.C.; Arbous, M.S.; de Man, A.M.S.; Beurskens, C.J.; Vroom, M.B.; Juffermans, N.P. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: A prospective substudy of a randomized trial. Crit. Care 2015, 19, 163. [Google Scholar] [CrossRef]
- Saoraya, J.; Wongsamita, L.; Srisawat, N.; Musikatavorn, K. Plasma syndecan-1 is associated with fluid requirements and clinical outcomes in emergency department patients with sepsis. Am. J. Emerg. Med. 2021, 42, 83–89. [Google Scholar] [CrossRef]
- Fisher, J.; Douglas, J.J.; Linder, A.; Boyd, J.H.; Walley, K.R.; Russell, J.A. Elevated Plasma Angiopoietin-2 Levels Are Associated With Fluid Overload, Organ Dysfunction, and Mortality in Human Septic Shock. Crit. Care Med. 2016, 44, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.; Shabanova, V.; Canarie, M.; Pinto, M.; da Silva, Y.S.; Bhandari, V.; Giuliano, J. Angiopoietin Level Trajectories in Toddlers With Severe Sepsis and Septic Shock and Their Effect on Capillary Endothelium. Shock 2019, 51, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Fiusa, M.M.L.; Costa-Lima, C.; De Souza, G.R.; Vigorito, A.C.; Aranha, F.J.P.; Lorand-Metze, I.; Annichino-Bizzacchi, J.M.; A De Souza, C.; De Paula, E.V. A high angiopoietin-2/angiopoietin-1 ratio is associated with a high risk of septic shock in patients with febrile neutropenia. Crit. Care 2013, 17, R169. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-M.; Wang, Y.-M.; Lin, H.-C.; Lee, K.-Y.; Huang, C.-D.; Liu, C.-Y.; Wang, C.-H.; Kuo, H.-P. Serum thrombomodulin level relates to the clinical course of disseminated intravascular coagulation, multiorgan dysfunction syndrome, and mortality in patients with sepsis*. Crit. Care Med. 2008, 36, 683–689. [Google Scholar] [CrossRef]
- Mihajlovic, D.M.; Lendak, D.F.; Draskovic, B.G.; Mikic, A.S.N.; Mitic, G.P.; Cebovic, T.N.; Brkic, S.V. Thrombomodulin is a Strong Predictor of Multiorgan Dysfunction Syndrome in Patients With Sepsis. Clin. Appl. Thromb. 2013, 21, 469–474. [Google Scholar] [CrossRef]
- Stahl, K.; Hillebrand, U.C.; Kiyan, Y.; Seeliger, B.; Schmidt, J.J.; Schenk, H.; Pape, T.; Schmidt, B.M.W.; Welte, T.; Hoeper, M.M.; et al. Effects of therapeutic plasma exchange on the endothelial glycocalyx in septic shock. Intensive Care Med. Exp. 2021, 9, 57. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef]
- Martin, L.B.; De Santis, R.; Koczera, P.; Simons, N.; Haase, H.; Heinbockel, L.; Brandenburg, K.; Marx, G.; Schuerholz, T. The Synthetic Antimicrobial Peptide 19-2.5 Interacts with Heparanase and Heparan Sulfate in Murine and Human Sepsis. PLoS ONE 2015, 10, e0143583. [Google Scholar] [CrossRef]
- Lee, W.L.; Slutsky, A.S. Sepsis and Endothelial Permeability. N. Engl. J. Med. 2010, 363, 689–691. [Google Scholar] [CrossRef]
- Geven, C.; Pickkers, P. The mechanism of action of the adrenomedullin-binding antibody adrecizumab. Crit. Care 2018, 22, 159. [Google Scholar] [CrossRef]
- Geven, C.; Peters, E.; Schroedter, M.; Struck, J.; Bergmann, A.; McCook, O.; Radermacher, P.; Kox, M.; Pickkers, P. Effects of the humanized anti-adrenomedullin antibody Adrecizumab (HAM8101) on vascular barrier function and survival in rodent models of systemic inflammation and sepsis. Shock 2018, 50, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.; Wachter, U.; Vogt, J.A.; Scheuerle, A.; McCook, O.; Weber, S.; Groger, M.; Stahl, B.; Georgieff, M.; Moller, P.; et al. Adrenomedullin binding improves catecholamine responsiveness and kidney function in resuscitated murine septic shock. Intensive Care Med. Exp. 2013, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Geven, C.; van Lier, D.; Blet, A.; Peelen, R.; Ten Elzen, B.; Mebazaa, A.; Kox, M.; Pickkers, P. Safety, tolerability and pharmacokinetics/pharmacodynamics of the adrenomedullin antibody adrecizumab in a first-in-human study and during experimental human endotoxaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Laterre, P.F.; Pickkers, P.; Marx, G.; Wittebole, X.; Meziani, F.; Dugernier, T.; Huberlant, V.; Schuerholz, T.; François, B.; Lascarrou, J.B.; et al. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: The AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. 2021, 47, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Lapinsky, S.; Dial, S.; Arabi, Y.; Dodek, P.; Wood, G.; Ellis, P.; Guzman, J.; Marshall, J.; Parrillo, J.E.; et al. Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009, 35, 871–881. [Google Scholar] [CrossRef]
- Ma, S.; Evans, R.G.; Iguchi, N.; Tare, M.; Parkington, H.C.; Bellomo, R.; May, C.N.; Lankadeva, Y.R. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation 2019, 26, e12483. [Google Scholar] [CrossRef]
- Zarbock, A.; Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury revisited: Pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 2014, 20, 588–595. [Google Scholar] [CrossRef]
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef]
- Manrique-Caballero, C.L.; Del Rio-Pertuz, G.; Gomez, H. Sepsis-Associated Acute Kidney Injury. Crit. Care Clin. 2021, 37, 279–301. [Google Scholar] [CrossRef]
- Bouchard, J.; Acharya, A.; Cerda, J.; Maccariello, E.R.; Madarasu, R.C.; Tolwani, A.J.; Liang, X.; Fu, P.; Liu, Z.H.; Mehta, R.L. A Prospective International Multicenter Study of AKI in the Intensive Care Unit. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 1324–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. CJASN 2007, 2, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Schaub, J.A.; Heung, M. Precision Medicine in Acute Kidney Injury: A Promising Future? Am. J. Respir. Crit. Care Med. 2019, 199, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef] [PubMed]
- Koyner, J.L.; Shaw, A.D.; Chawla, L.S.; Hoste, E.A.; Bihorac, A.; Kashani, K.; Haase, M.; Shi, J.; Kellum, J.A.; Sapphire Investigators. Tissue Inhibitor Metalloproteinase-2 (TIMP-2)⋅IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J. Am. Soc. Nephrol. JASN 2015, 26, 1747–1754. [Google Scholar] [CrossRef]
- Meersch, M.; Schmidt, C.; Hoffmeier, A.; Van Aken, H.; Wempe, C.; Gerss, J.; Zarbock, A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med. 2017, 43, 1551–1561. [Google Scholar] [CrossRef]
- Zarbock, A.; Küllmar, M.; Ostermann, M.; Lucchese, G.; Baig, K.; Cennamo, A.; Rajani, R.; McCorkell, S.; Arndt, C.; Wulf, H.; et al. Prevention of Cardiac Surgery-Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 2021, 133, 292–302. [Google Scholar] [CrossRef]
- Göcze, I.; Jauch, D.; Götz, M.; Kennedy, P.; Jung, B.; Zeman, F.; Gnewuch, C.; Graf, B.M.; Gnann, W.; Banas, B.; et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann. Surg. 2018, 267, 1013–1020. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Biomarker-Guided Intervention to Prevent Acute Kidney Injury (BigpAK-2); National Library of Medicine: Bethesda, MD, USA, 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04647396 (accessed on 14 February 2022).
- Bhatraju, P.K.; Zelnick, L.R.; Herting, J.; Katz, R.; Mikacenic, C.; Kosamo, S.; Morrell, E.D.; Robinson-Cohen, C.; Calfee, C.S.; Christie, J.D.; et al. Identification of Acute Kidney Injury Subphenotypes with Differing Molecular Signatures and Responses to Vasopressin Therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 863–872. [Google Scholar] [CrossRef]
- Ricciuto, D.R.; dos Santos, C.C.; Hawkes, M.; Toltl, L.J.; Conroy, A.L.; Rajwans, N.; Lafferty, E.I.; Cook, D.J.; Fox-Robichaud, A.; Kahnamoui, K.; et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit. Care Med. 2011, 39, 702–710. [Google Scholar] [CrossRef]
- Robinson-Cohen, C.; Katz, R.; Price, B.L.; Harju-Baker, S.; Mikacenic, C.; Himmelfarb, J.; Liles, W.C.; Wurfel, M.M. Association of markers of endothelial dysregulation Ang1 and Ang2 with acute kidney injury in critically ill patients. Crit. Care 2016, 20, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, J.A.; Walley, K.R.; Singer, J.; Gordon, A.C.; Hébert, P.C.; Cooper, D.J.; Holmes, C.L.; Mehta, S.; Granton, J.T.; Storms, M.M.; et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N. Engl. J. Med. 2008, 358, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Cornell, T.T.; Wynn, J.; Shanley, T.P.; Wheeler, D.S.; Wong, H.R. Mechanisms and regulation of the gene-expression response to sepsis. Pediatrics 2010, 125, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Arcaroli, J.; Fessler, M.B.; Abraham, E. Genetic polymorphisms and sepsis. Shock 2005, 24, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Shidham, A.; Wong, H.R.; Khatri, P. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci. Transl. Med. 2015, 7, 287ra71. [Google Scholar] [CrossRef]
- Davenport, E.E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.; et al. Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet Respir. Med. 2016, 4, 259–271. [Google Scholar] [CrossRef]
- Kiryluk, K.; Bomback, A.S.; Cheng, Y.L.; Xu, K.; Camara, P.G.; Rabadan, R.; Sims, P.A.; Barasch, J. Precision Medicine for Acute Kidney Injury (AKI): Redefining AKI by Agnostic Kidney Tissue Interrogation and Genetics. Semin. Nephrol. 2018, 38, 40–51. [Google Scholar] [CrossRef]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids. From the VANISH Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef]
- Scicluna, B.P.; Baillie, J.K. The Search for Efficacious New Therapies in Sepsis Needs to Embrace Heterogeneity. Am. J. Respir. Crit. Care Med. 2019, 199, 936–938. [Google Scholar] [CrossRef]
- Scicluna, B.P.; van Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nürnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L.; et al. Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet Respir. Med. 2017, 5, 816–826. [Google Scholar] [CrossRef]

| Immunological Biomarkers | |
|---|---|
| C-reactive protein (CRP) | Indicates acute systemic inflammation [38] Screening for early onset neonatal sepsis [39] (Predict survival in patients with sepsis) [40] |
| Procalcitonin (PCT) | Diagnosis of sepsis [41,42] Suggest bacterial infection [43] Monitor treatment response to antibiotics and guide cessation of antibiotic treatment [44,45,46,47] |
| Presepsin (soluble CD14) | Early detection of sepsis (earlier increase than PCT and CRP) [48] Monitor host response [49] Higher in patients with bacterial infection [50] May be combined with other biomarkers in a panel [51] No validity in patients with acute kidney injury [52] |
| Interleukin-6 (IL-6) | Early detection of sepsis [53,54] Early detection of SIRS [55] Differentiate infectious from sterile SIRS [56] |
| Interleukin-8 (IL-8) | Diagnosis of sepsis [57] |
| CD64 expression on neutrophils (nCD64) | Early detection of sepsis [58,59,60,61,62,63,64] (monitoring of sepsis) [63,65] Prognostic marker of sepsis [66,67] |
| Soluble programmed death ligand 1 (sPD-L1) | Detect immunosuppressed states in sepsis patients [68,69] Potential therapeutic target [70] |
| HLA-DR expression on antigen-presenting cells | High HLA-DR expression: Detect hyperinflammatory state [71] Low HLA-DR expression: Detect immunosuppressed state [72] Predict poor survival in patients with septic shock [73] Potential biomarker for enrichment of clinical trials of sepsis [74] |
| Pentraxin (PTX-3) | Assessment of septic shock severity [75,76] Prediction of mortality in patients with sepsis or septic shock [77] |
| Complement protein 5a (C5a) | Limited utility in sepsis due to both pro- and anti-inflammatory effects [78,79] |
| Infectious biomarkers | |
| Lipopolysaccharide-binding protein (LPS-bp) | Discriminate sterile from infectious basis of SIRS or sepsis [80] |
| Pathogen-associated molecular patterns (PAMPs) | Early detection of pathogen-based immune stimuli [81,82,83,84] |
| Biomarkers of endothelial or glycocalyx dysfunction | |
| Syndecan-1 | Assessment of endothelial barrier dysfunction in sepsis [85] Predict organ failure due to endothelial dysfunction [86] Prediction of DIC or coagulatory dysfunction in sepsis-associated endothelial dysfunction [87,88,89] (May be helpful to guide fluid resuscitation in early sepsis) [90] |
| Adrenomedullin (ADM) | s. below (use case no. 2) |
| Angiopoietin-1, -2 | Detect fluid overload and endothelial leakage in sepsis [91,92] Predict septic shock [93] |
| Thrombomodulin | Predict multi organ failure and DIC in sepsis [94,95] |
| Heparanase-1 and -2 (Hpa-1, Hpa-2) | May identify septic patients with potential benefit from therapeutic plasma exchange therapy [96] Potential therapeutic target [97,98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Groote, T.; Meersch-Dini, M. Biomarkers for the Prediction and Judgement of Sepsis and Sepsis Complications: A Step towards precision medicine? J. Clin. Med. 2022, 11, 5782. https://doi.org/10.3390/jcm11195782
von Groote T, Meersch-Dini M. Biomarkers for the Prediction and Judgement of Sepsis and Sepsis Complications: A Step towards precision medicine? Journal of Clinical Medicine. 2022; 11(19):5782. https://doi.org/10.3390/jcm11195782
Chicago/Turabian Stylevon Groote, Thilo, and Melanie Meersch-Dini. 2022. "Biomarkers for the Prediction and Judgement of Sepsis and Sepsis Complications: A Step towards precision medicine?" Journal of Clinical Medicine 11, no. 19: 5782. https://doi.org/10.3390/jcm11195782
APA Stylevon Groote, T., & Meersch-Dini, M. (2022). Biomarkers for the Prediction and Judgement of Sepsis and Sepsis Complications: A Step towards precision medicine? Journal of Clinical Medicine, 11(19), 5782. https://doi.org/10.3390/jcm11195782






