Shedding of the Endothelial Glycocalyx Independent of Systemic Tryptase Release during Oncologic Oral Surgery: An Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Registration
2.3. Patient Recruitment
2.4. Measurement of Glycocalyx Shedding
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
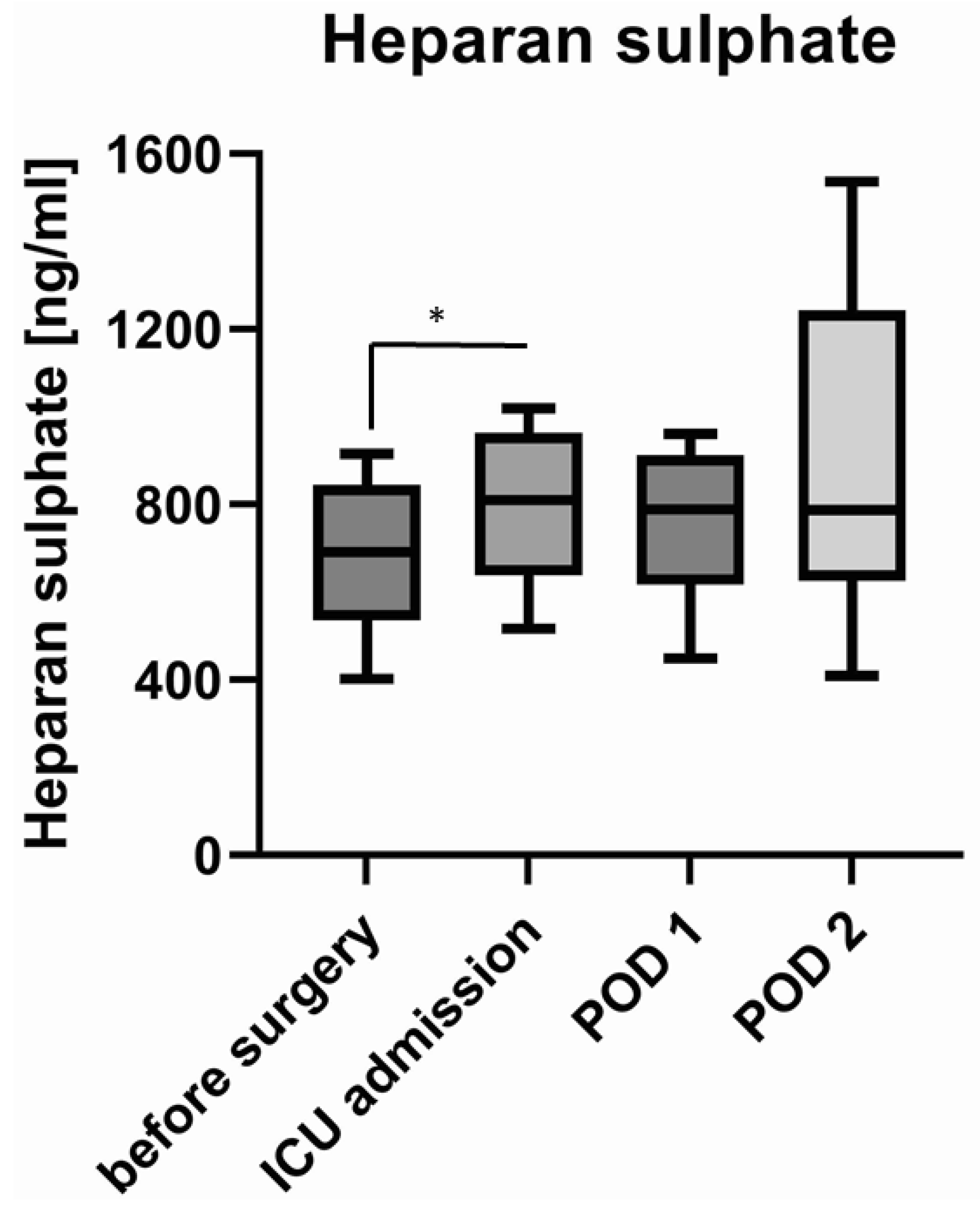
3.2. Shedding of Glycocalyx Components
3.3. Concentration of Tryptase
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef]
- Hahn, R.G.; Patel, V.; Dull, R.O. Human glycocalyx shedding: Systematic review and critical appraisal. Acta Anaesthesiol. Scand. 2021, 65, 590–606. [Google Scholar] [CrossRef]
- Bogner-Flatz, V.; Braunstein, M.; Ocker, L.E.; Kusmenkov, T.; Tschoep, J.; Ney, L.; Böcker, W.; Annecke, T. On-the-Scene Hyaluronan and Syndecan-1 Serum Concentrations and Outcome after Cardiac Arrest and Resuscitation. Mediat. Inflamm. 2019, 2019, 8071619. [Google Scholar] [CrossRef]
- Chappell, D.; Bruegger, D.; Potzel, J.; Jacob, M.; Brettner, F.; Vogeser, M.; Conzen, P.; Becker, B.F.; Rehm, M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit. Care. 2014, 18, 538. [Google Scholar] [CrossRef]
- Rehm, M.; Bruegger, D.; Christ, F.; Conzen, P.; Thiel, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Reichart, B.; et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007, 116, 1896–1906. [Google Scholar] [CrossRef]
- Annecke, T.; Fischer, J.; Hartmann, H.; Tschoep, J.; Rehm, M.; Conzen, P.; Sommerhoff, C.; Becker, B. Shedding of the coronary endothelial glycocalyx: Effects of hypoxia/reoxygenation vs. ischaemia/reperfusion. Br. J. Anaesth. 2011, 107, 679–686. [Google Scholar] [CrossRef]
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402. [Google Scholar] [CrossRef]
- Farwell, D.G.; Reilly, D.F.; Weymuller, E.A., Jr.; Greenberg, D.L.; Staiger, T.O.; Futran, N.A. Predictors of perioperative complications in head and neck patients. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 505–511. [Google Scholar] [CrossRef]
- Tapia, B.; Garrido, E.; Cebrian, J.L.; Del Castillo, J.L.; Alsina, E.; Gilsanz, F. New techniques and recommendations in the management of free flap surgery for head and neck defects in cancer patients. Minerva Anestesiol. 2020, 86, 861–871. [Google Scholar] [CrossRef]
- Arthur, A.; McCall, P.J.; Jolly, L.; Kinsella, J.; Kirk, A.; Shelley, B.G. Endothelial glycocalyx layer shedding following lung resection. Biomark. Med. 2016, 10, 1033–1038. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef]
- Holzmann, M.S.; Winkler, M.S.; Strunden, M.S.; Izbicki, J.R.; Schoen, G.; Greiwe, G.; Pinnschmidt, O.H.; Poppe, A.; Saugel, B.; Daum, G.; et al. Syndecan-1 as a biomarker for sepsis survival after major abdominal surgery. Biomark. Med. 2018, 12, 119–127. [Google Scholar] [CrossRef]
- Bruegger, D.; Rehm, M.; Abicht, J.; Paul, J.O.; Stoeckelhuber, M.; Pfirrmann, M.; Reichart, B.; Becker, B.F.; Christ, F. Shedding of the endothelial glycocalyx during cardiac surgery: On-pump versus off-pump coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 1445–1447. [Google Scholar] [CrossRef]
- Dekker, N.A.M.; Veerhoek, D.; Koning, N.J.; van Leeuwen, A.L.I.; Elbers, P.W.G.; Brom, C.E.V.D.; Vonk, A.B.A.; Boer, C. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 2019, 74, 609–618. [Google Scholar] [CrossRef]
- Schiefer, J.; Faybik, P.; Koch, S.; Tudor, B.; Kollmann, D.; Kuessel, L.; Krenn, C.G.; Berlakovich, G.; Baron, D.M.; Baron-Stefaniak, J. Glycocalyx Damage Within Human Liver Grafts Correlates with Graft Injury and Postoperative Graft Function after Orthotopic Liver Transplantation. Transplantation 2020, 104, 72–78. [Google Scholar] [CrossRef]
- Steppan, J.; Hofer, S.; Funke, B.; Brenner, T.; Henrich, M.; Martin, E.; Weitz, J.; Hofmann, U.; Weigand, M.A. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J. Surg. Res. 2011, 165, 136–141. [Google Scholar] [CrossRef]
- Casanova, J.; Simon, C.; Vara, E.; Sanchez, G.; Rancan, L.; Abubakra, S.; Calvo, A.; Gonzalez, F.J.; Garutti, I. Sevoflurane anesthetic preconditioning protects the lung endothelial glycocalyx from ischemia reperfusion injury in an experimental lung autotransplant model. J. Anesth. 2016, 30, 755–762. [Google Scholar] [CrossRef]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Yang, X.; Meegan, J.E.; Jannaway, M.; Coleman, D.C.; Yuan, S.Y. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc. Res. 2018, 114, 1752–1763. [Google Scholar] [CrossRef]
- Osuka, A.; Kusuki, H.; Yoneda, K.; Matsuura, H.; Matsumoto, H.; Ogura, H.; Ueyama, M. Glycocalyx Shedding is Enhanced by Age and Correlates with Increased Fluid Requirement in Patients with Major Burns. Shock 2018, 50, 60–65. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, E.J.; Lee, H.J.; Min, J.Y.; Kim, T.W.; Choi, E.C.; Kim, W.S.; Koo, B.-N. Effect of goal-directed haemodynamic therapy in free flap reconstruction for head and neck cancer. Acta Anaesthesiol. Scand. 2018, 62, 903–914. [Google Scholar] [CrossRef]
- Tapia, B.; Garrido, E.; Cebrian, J.L.; Del Castillo, J.; Gonzalez, J.; Losantos, I.; Gilsanz, F. Impact of Goal Directed Therapy in Head and Neck Oncological Surgery with Microsurgical Reconstruction: Free Flap Viability and Complications. Cancers 2021, 13, 1545. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef]
- Fuchs, A.; Neumann, T.; Drinhaus, H.; Herrmann, A.; Vink, H.; Annecke, T. Effects of a single aerobic exercise on perfused boundary region and microvascular perfusion: A field study. J. Clin. Monit. Comput. 2022, 36, 371–377. [Google Scholar] [CrossRef]
- Gao, W.; Fang, F.; Xia, T.J.; Zhang, Y.; Sun, J.; Wu, Q.; Wang, W. Doxycycline can reduce glycocalyx shedding by inhibiting matrix metalloproteinases in patients undergoing cardiopulmonary bypass: A randomized controlled trial. Microvasc. Res. 2022, 142, 104381. [Google Scholar] [CrossRef]


| Age [years] | 64 (55–74) |
| Sex [number (%)] | female 4 (25%)/male 12 (75%) |
| weight [kg] | 80 (65–90) |
| Height [m] | 177 (169–180) |
| Diagnosis [number] | Squamous cell carcinoma of |
| maxilla n = 2 | |
| cheek n = 3 | |
| tongue n = 5 | |
| base of the mouth n = 3 | |
| palate n = 2 | |
| Synovial sarcoma maxilla n = 1 | |
| Type of surgery [number] | Radial free flap n = 9 |
| Fibular free flap n = 3 | |
| Scapular free flap n = 1 | |
| Latissimus dorsi free flap n = 1 | |
| Anterolateral thigh flap n = 1 | |
| Skin graft n = 1 |
| Duration of Surgery [min] | 480 (393–570) |
| Crystalloid infusion [mL] | 5550 (4750–7000) |
| Colloid infusion [mL] | 0 (0–500) |
| Red blood cell transfusion [mL] | 177 (169–180) |
| Fresh frozen plasma transfusion [mL] | 0 (0, 0) |
| Blood loss [mL] | 1150 (650–1575) |
| Diuresis [mL] | 1600 (1325–1893) |
| Maximum norepinephrine [mcg/kg/min] | 0.12 (0.07–0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drinhaus, H.; Schroeder, D.C.; Hunzelmann, N.; Herff, H.; Annecke, T.; Böttiger, B.W.; Wetsch, W.A. Shedding of the Endothelial Glycocalyx Independent of Systemic Tryptase Release during Oncologic Oral Surgery: An Observational Study. J. Clin. Med. 2022, 11, 5797. https://doi.org/10.3390/jcm11195797
Drinhaus H, Schroeder DC, Hunzelmann N, Herff H, Annecke T, Böttiger BW, Wetsch WA. Shedding of the Endothelial Glycocalyx Independent of Systemic Tryptase Release during Oncologic Oral Surgery: An Observational Study. Journal of Clinical Medicine. 2022; 11(19):5797. https://doi.org/10.3390/jcm11195797
Chicago/Turabian StyleDrinhaus, Hendrik, Daniel C. Schroeder, Nicolas Hunzelmann, Holger Herff, Thorsten Annecke, Bernd W. Böttiger, and Wolfgang A. Wetsch. 2022. "Shedding of the Endothelial Glycocalyx Independent of Systemic Tryptase Release during Oncologic Oral Surgery: An Observational Study" Journal of Clinical Medicine 11, no. 19: 5797. https://doi.org/10.3390/jcm11195797






