Transient Global Amnesia (TGA): Sex-Specific Differences in Blood Pressure and Cerebral Microangiopathy in Patients with TGA
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Control Group
2.3. Procedure
2.4. Data Analysis
3. Results
3.1. Demographic Characteristics
3.2. Vascular Risk Factors
3.3. Comparison with Acute Stroke Patients
3.4. TGA Recurrences
3.5. MR Imaging Abnormalities
3.6. Comparison with Acute Stroke Patients
4. Discussion
4.1. Sex Distribution
4.2. Cholesterol Levels
4.3. C-Reactive Protein
4.4. DWI Lesions
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| DWI | diffusion-weighted imaging |
| MRI | magnetic resonance imaging |
| TGA | transient global amnesia |
References
- Bender, M.B. Syndrome of Isolated Episode of Confusion with Amnesia. J. Hillside Hosp. 1956, 5, 212–215. [Google Scholar]
- Shad, K.F.; Dogan, K.H. (Eds.) Neurocognitive Perspective of Transient Global Amnesia; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-83962-974-7. [Google Scholar]
- Sparaco, M.; Pascarella, R.; Muccio, C.F.; Zedde, M. Forgetting the Unforgettable: Transient Global Amnesia Part I: Pathophysiology and Etiology. J. Clin. Med. 2022, 11, 3373. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, B.I.; Kim, S.E. Is Transient Global Amnesia a Network Disease? Eur. Neurol. 2018, 80, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Raptopoulou, M.; Mpourlios, S.; Siokas, V.; Tsouris, Z.; Aloizou, A.-M.; Dastamani, M.; Brotis, A.; Bogdanos, D.; Xiromerisiou, G.; et al. Factors associated with recurrent transient global amnesia: Systematic review and pathophysiological insights. Rev. Neurosci. 2021, 32, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Rogalewski, A.; Beyer, A.; Friedrich, A.; Plümer, J.; Zuhorn, F.; Klingebiel, R.; Woermann, F.G.; Bien, C.G.; Greeve, I.; Schäbitz, W.-R. Transient Global Amnesia (TGA): Younger Age and Absence of Cerebral Microangiopathy Are Potentially Predisposing Factors for TGA Recurrence. Front. Neurol. 2021, 12, 736563. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E. The Role of Sex in Memory Function: Considerations and Recommendations in the Context of Exercise. J. Clin. Med. 2018, 7, 132. [Google Scholar] [CrossRef]
- Herlitz, A.; Nilsson, L.-G.; Bäckman, L. Gender differences in episodic memory. Mem. Cogn. 1997, 25, 801–811. [Google Scholar] [CrossRef]
- Fujita, F.; Diener, E.; Sandvik, E. Gender differences in negative affect and well-being: The case for emotional intensity. J. Pers. Soc. Psychol. 1991, 61, 427–434. [Google Scholar] [CrossRef]
- Seidlitz, L.; Diener, E. Sex differences in the recall of affective experiences. J. Pers. Soc. Psychol. 1998, 74, 262–271. [Google Scholar] [CrossRef]
- Rentz, D.M.; Weiss, B.K.; Jacobs, E.G.; Cherkerzian, S.; Klibanski, A.; Remington, A.; Aizley, H.; Goldstein, J.M. Sex differences in episodic memory in early midlife: Impact of reproductive aging. Menopause 2017, 24, 400–408. [Google Scholar] [CrossRef]
- Nyberg, L.; Habib, R.; Herlitz, A. Brain activation during episodic memory retrieval: Sex differences. Acta Psychol. 2000, 105, 181–194. [Google Scholar] [CrossRef]
- Filipek, P.A.; Richelme, C.; Kennedy, D.N.; Caviness, J.V.S. The Young Adult Human Brain: An MRI-based Morphometric Analysis. Cereb. Cortex 1994, 4, 344–360. [Google Scholar] [CrossRef]
- Paus, T.; Otaky, N.; Caramanos, Z.; Macdonald, D.; Zijdenbos, A.; D’Avirro, D.; Gutmans, D.; Holmes, C.; Tomaiuolo, F.; Evans, A.C. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: Hemispheric asymmetries, gender differences and probability maps. J. Comp. Neurol. 1996, 376, 664–673. [Google Scholar] [CrossRef]
- Hoyer, C.; Ebert, A.; Sandikci, V.; Platten, M.; Szabo, K. Sex-related differences in stressful events precipitating transient global amnesia—A retrospective observational study. J. Neurol. Sci. 2021, 425, 117464. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Gentile, M.; Fassetta, G.; Casetta, I.; Caneve, G. Incidence of transient global amnesia in the Belluno province, Italy: 1985 through 1995. Acta Neurol. Scand. 1997, 95, 303–310. [Google Scholar] [CrossRef]
- Romoli, M.; Tuna, M.A.; McGurgan, I.; Li, L.; Giannandrea, D.; Eusebi, P.; Caprioli, F.T.; Lotti, A.; Salvadori, N.; Sarchielli, P.; et al. Long-Term Risk of Stroke After Transient Global Amnesia in Two Prospective Cohorts. Stroke 2019, 50, 2555–2557. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.P.; Ferro, J.M.; Ferro, H. Transient global amnesia: A Case Control Study. Brain 1992, 115, 261–270. [Google Scholar] [CrossRef]
- Quinette, P.; Guillery-Girard, B.; Dayan, J.; De La Sayette, V.; Marquis, S.; Viader, F.; Desgranges, B.; Eustache, F. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain 2006, 129, 1640–1658. [Google Scholar] [CrossRef]
- Liampas, I.; Raptopoulou, M.; Siokas, V.; Bakirtzis, C.; Tsouris, Z.; Aloizou, A.-M.; Dastamani, M.; Brotis, A.; Bogdanos, D.; Dardiotis, E. Conventional cardiovascular risk factors in Transient Global Amnesia: Systematic review and proposition of a novel hypothesis. Front. Neuroendocrinol. 2021, 61, 100909. [Google Scholar] [CrossRef]
- Rogalewski, A.; Beyer, A.; Friedrich, A.; Plümer, J.; Zuhorn, F.; Greeve, I.; Klingebiel, R.; Woermann, F.G.; Bien, C.G.; Schäbitz, W.-R. Transient Global Amnesia (TGA): Influence of Acute Hypertension in Patients Not Adapted to Chronic Hypertension. Front. Neurol. 2021, 12, 666632. [Google Scholar] [CrossRef]
- Hodges, J.R.; Warlow, C.P. Syndromes of transient amnesia: Towards a classification. A study of 153 cases. J. Neurol. Neurosurg. Psychiatry 1990, 53, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Karten, Analysen und Statistiken zur ansässigen Bevölkerung. Available online: https://ugeo.urbistat.com/AdminStat/de/de/demografia/eta/bielefeld%2C-kreisfreie-stadt/5711/3 (accessed on 12 December 2021).
- Bland, J.M.; Altman, D.G. Multiple significance tests: The Bonferroni method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; O’Donnell, M.; Lamelas, P.; Teo, K.; Rangarajan, S.; Yusuf, S. Physical Activity and Anger or Emotional Upset as Triggers of Acute Myocardial Infarction: The INTERHEART Study. Circulation 2016, 134, 1059–1067. [Google Scholar] [CrossRef]
- Vaccarino, V.; Shah, A.; Rooks, C.; Ibeanu, I.; Nye, J.A.; Pimple, P.; Salerno, A.; D’Marco, L.; Karohl, C.; Bremner, J.D.; et al. Sex Differences in Mental Stress–Induced Myocardial Ischemia in Young Survivors of an Acute Myocardial Infarction. Psychosom. Med. 2014, 76, 171–180. [Google Scholar] [CrossRef]
- Almuwaqqat, Z.; Sullivan, S.; Hammadah, M.; Lima, B.B.; Shah, A.; Abdelhadi, N.; Fang, S.; Wilmot, K.; Al Mheid, I.; Bremner, J.D.; et al. Sex-Specific Association Between Coronary Artery Disease Severity and Myocardial Ischemia Induced by Mental Stress. Psychosom. Med. 2019, 81, 57–66. [Google Scholar] [CrossRef]
- Elsaid, N.; Saied, A.; Kandil, H.; Soliman, A.; Taher, F.; Hadi, M.; Giridharan, G.; Jennings, R.; Casanova, M.; Keynton, R.; et al. Impact of Stress and Hypertension on the Cerebrovasculature. Front. Biosci. 2021, 26, 1643–1652. [Google Scholar] [CrossRef]
- Burrage, E.; Marshall, K.; Santanam, N.; Chantler, P. Cerebrovascular Dysfunction with Stress and Depression. Brain Circ. 2018, 4, 43. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Heuser, I.J.; Gotthardt, U.; Schweiger, U.; Schmider, J.; Lammers, C.-H.; Dettling, M.; Holsboer, F. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: Importance of gender. Neurobiol. Aging 1994, 15, 227–231. [Google Scholar] [CrossRef]
- Sanchez-Cano, F.; Hernández-Kelly, L.C.; Ortega, A. The Blood–Brain Barrier: Much More Than a Selective Access to the Brain. Neurotox. Res. 2021, 39, 2154–2174. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; Fargo, A.E.; Leach, C.N., Jr.; Scherzer, H.H. Short-term effect of dynamic exercise on arterial blood pressure. Circulation 1991, 83, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Howland, J.G.; Wang, Y.T. Chapter 8 Synaptic Plasticity in Learning and Memory: Stress Effects in the Hippocampus. Prog. Brain Res. 2008, 169, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Raptopoulou, M.; Siokas, V.; Tsouris, Z.; Brotis, A.; Aloizou, A.-M.; Dastamani, M.; Dardiotis, E. The long-term prognosis of Transient Global Amnesia: A systematic review. Rev. Neurosci. 2021, 32, 531–543. [Google Scholar] [CrossRef]
- Sparaco, M.; Pascarella, R.; Muccio, C.F.; Zedde, M. Forgetting the Unforgettable: Transient Global Amnesia Part II: A Clinical Road Map. J. Clin. Med. 2022, 11, 3940. [Google Scholar] [CrossRef]
- Waliszewska-Prosol, M.; Nowakowska-Kotas, M.; Bladowska, J.; Papier, P.; Budrewicz, S.; Pokryszko-Dragan, A. Transient Global Amnesia—Risk Factors and Putative Background. Neurol. India 2020, 68, 624–629. [Google Scholar] [CrossRef]
- Yu, A.Y.X.; Penn, A.M.; Lesperance, M.L.; Croteau, N.S.; Balshaw, R.F.; Votova, K.; Bibok, M.B.; Penn, M.; Saly, V.; Hegedus, J.; et al. Sex Differences in Presentation and Outcome After an Acute Transient or Minor Neurologic Event. JAMA Neurol. 2019, 76, 962–968. [Google Scholar] [CrossRef]
- Himeno, T.; Kuriyama, M.; Takemaru, M.; Kanaya, Y.; Shiga, Y.; Takeshima, S.; Takamatsu, K.; Shimoe, Y.; Fukushima, T.; Matsubara, E. Vascular Risk Factors and Internal Jugular Venous Flow in Transient Global Amnesia: A Study of 165 Japanese Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 2272–2278. [Google Scholar] [CrossRef]
- Jang, J.-W.; Park, S.Y.; Hong, J.-H.; Park, Y.H.; Kim, J.E.; Kim, S. Different Risk Factor Profiles between Transient Global Amnesia and Transient Ischemic Attack: A Large Case-Control Study. Eur. Neurol. 2014, 71, 19–24. [Google Scholar] [CrossRef]
- Lauria, G.; Gentile, M.; Fassetta, G.; Casetta, I.; Caneve, G. Transient global amnesia and transient ischemic attack: A community-based case-control study. Acta Neurol. Scand. 1998, 97, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L.; Bertini, E.; LaMassa, M.; Pracucci, G.; Inzitari, D. Clinical features, risk factors, and prognosis in transient global amnesia: A follow-up study. Eur. J. Neurol. 2005, 12, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Winbeck, K.; Etgen, T.; Von Einsiedel, H.G.; Röttinger, M.; Sander, D. DWI in transient global amnesia and TIA: Proposal for an ischaemic origin of TGA. J. Neurol. Neurosurg. Psychiatry 2005, 76, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Zorzon, M.; Antonutti, L.; Maseè, G.; Biasutti, E.; Vitrani, B.; Cazzato, G. Transient Global Amnesia and Transient Ischemic Attack: Natural History, Vascular Risk Factors, and Associated Conditions. Stroke 1995, 26, 1536–1542. [Google Scholar] [CrossRef]
- Akkawi, N.M.; Agosti, C.; Anzola, G.P.; Borroni, B.; Magoni, M.; Pezzini, A.; Rozzini, L.; Vignolo, L.A.; Padovani, A. Transient Global Amnesia: A Clinical and Sonographic Study. Eur. Neurol. 2003, 49, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.R.; Warlow, C.P. The aetiology of transient global amnesia: A case-control study of 114 cases with prospective follow-up. Brain 1990, 113, 639–657. [Google Scholar] [CrossRef]
- Tharu, B.P.; Tsokos, C.P. A Statistical Study of Serum Cholesterol Level by Gender and Race. J. Res. Health Sci. 2017, 17, e00386. [Google Scholar]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Rogowski, O.; Zeltser, D.; Shapira, I.; Burke, M.; Zakut, V.; Mardi, T.; Ben-Assayag, E.; Serov, J.; Rozenblat, M.; Berliner, S. Gender difference in C-reactive protein concentrations in individuals with atherothrombotic risk factors and apparently healthy ones. Biomarkers 2004, 9, 85–92. [Google Scholar] [CrossRef]
- Ridker, P.M. Cardiology Patient Page. C-reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation 2003, 108, e81–e85. [Google Scholar] [CrossRef]


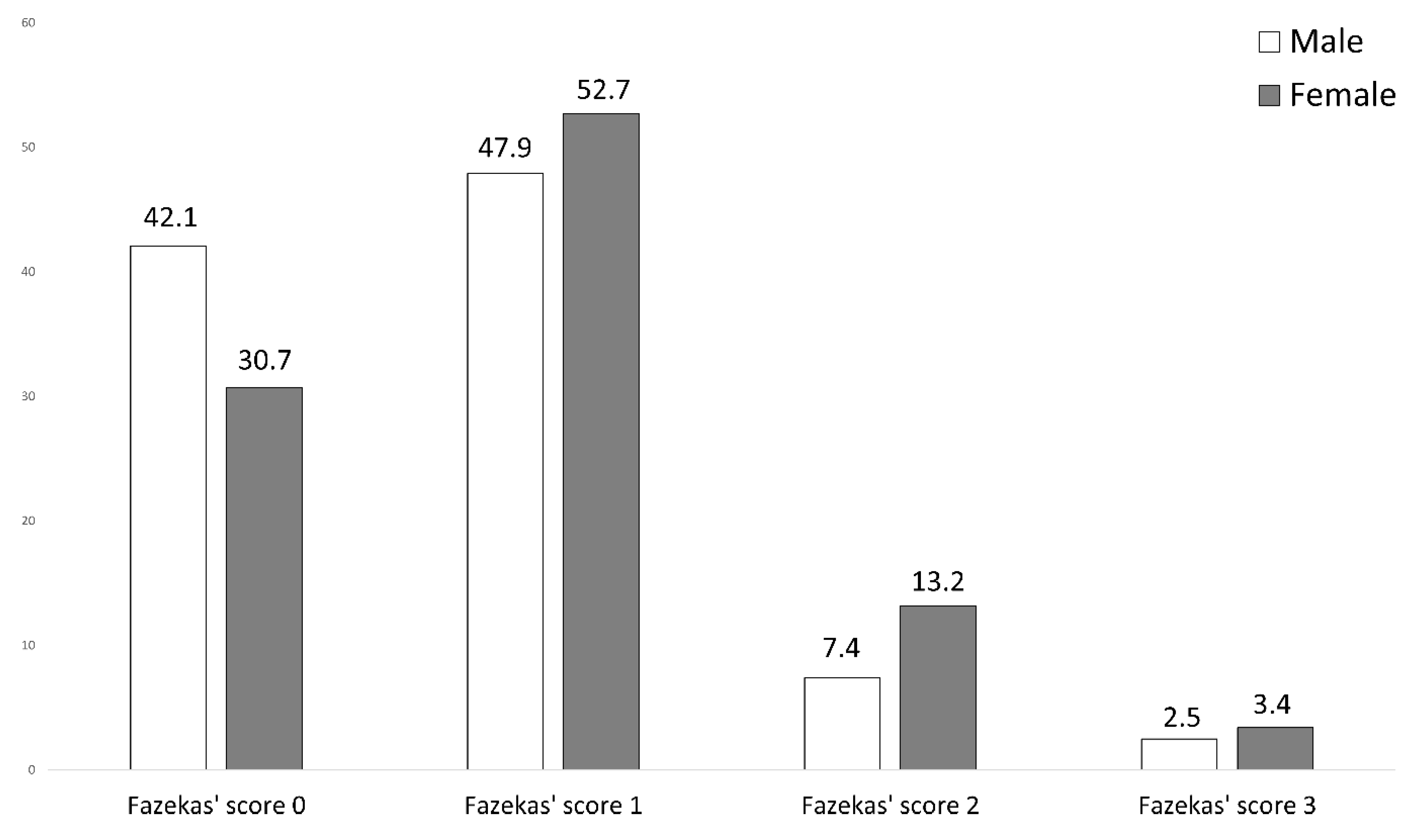
| Female TGA Patients (n = 230) | Male TGA Patients (n = 142) | Test Statistics | |
|---|---|---|---|
| Age | 68.1 ± 9.6 | 65.9 ± 11.4 | U = 17,720.500, Z = 1.381, p = 0.167 c |
| Hypertension | 161/228 (70.6%) | 101/140 (72.6%) | χ2 = 0.099, p = 0.753 a |
| Systolic blood pressure on admission | 173.2 ± 23.4 mm Hg | 165.8 ± 22.0 mm Hg | T = −2.725, p = 0.014 b |
| Diastolic blood pressure on admission | 93.0 ± 13.0 mm Hg | 92.3 ± 13.6 mm Hg | T = −0.407, p = 0.684 b |
| Diabetes mellitus | 12/228 (5.3%) | 8/142 (5.6%) | χ2 = 0.024, p = 0.878 a |
| Serum glucose level on admission | 117.5 ± 19.2 mg/dL | 116.3 ± 23.1 mg/dL | U = 14,335.500, Z = 1.257, p = 0.209 c |
| HbA1c | 5.6 ± 0.5 % | 5.5 ± 0.7 % | U = 11,590.500, Z = 1.669, p = 0.095 c |
| Hypercholesterolemia (>200 mg/dL at admission) | 140/202 (69.3%) | 62/118 (52.2%) | χ2 = 8.994, p = 0.003 a |
| Serum cholesterol level on admission | 221.6 ± 40.7 mg/dL | 207.6 ± 45.5 mg/dL | T = −2.800, p = 0.005 b |
| CRP level on admission | 2.8 ± 6.4 mg/L | 1.7 ± 1.8 mg/L | U = 16,317.500, Z = 2.530, p = 0.011 c |
| LVEF < 50% | This comparison could not be computed, as only 3 TGA patients displayed LVEF < 50% (1 female, 2 male). | ||
| Septal hypertrophy (male >10 mm, female > 9 mm) | 66/96 (68.8%) | 31/44 (70.5%) | χ2 = 0.041, p = 0.839 a |
| Cerebral stenosis | 17/210 (8.1%) | 6/127 (4.7%) | χ2 = 1.414, p = 0.234 a |
| Atrial fibrillation | 21/228 (9.2%) | 8/142 (5.6%) | χ2 = 1.550, p = 0.213 a |
| CHA2DS2-VASc score (corrected for sex) | 2.1 ± 1.5 | 2.1 ± 1.5 | U = 16,022.500, Z = −0.312, p = 0.755 c |
| Presence of DWI lesion | 101/205 (49.3%) | 61/122 (50.0%) | χ2 = 0.016, p = 0.898 a |
| Unilateral vs. bilateral lesion in case of presence of DWI lesion | Bilateral 26/101 (25.7%) | Bilateral 13/61 (21.3%) | χ2 = 0.409, p = 0.523 a |
| Antiplatelet therapy at discharge | 129/229 (56.3%) | 85/142 (59.9%) | χ2 = 0.447, p = 0.504 a |
| OAC at discharge | 19/229 (8.3%) | 11/142 (7.7%) | χ2 = 0.036, p = 0.850 a |
| Statin therapy at discharge | 120/229 (52.4%) | 77/142 (54.2%) | χ2 = 0.117, p = 0.732 a |
| Antihypertensive drugs at discharge | 159/229 (69.4%) | 102/142 (71.8%) | χ2 = 0.242, p = 0.623 a |
| Former stroke | 28/229 (12.2%) | 20/142 (14.1%) | χ2 = 0.268, p = 0.604 a |
| Recurrence | 19/230 (8.3%) | 10/142 (7.0%) | χ2 = 0.181, p = 0.670 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalewski, A.; Beyer, A.; Friedrich, A.; Zuhorn, F.; Klingebiel, R.; Woermann, F.G.; Oertelt-Prigione, S.; Schäbitz, W.-R. Transient Global Amnesia (TGA): Sex-Specific Differences in Blood Pressure and Cerebral Microangiopathy in Patients with TGA. J. Clin. Med. 2022, 11, 5803. https://doi.org/10.3390/jcm11195803
Rogalewski A, Beyer A, Friedrich A, Zuhorn F, Klingebiel R, Woermann FG, Oertelt-Prigione S, Schäbitz W-R. Transient Global Amnesia (TGA): Sex-Specific Differences in Blood Pressure and Cerebral Microangiopathy in Patients with TGA. Journal of Clinical Medicine. 2022; 11(19):5803. https://doi.org/10.3390/jcm11195803
Chicago/Turabian StyleRogalewski, Andreas, Anne Beyer, Anja Friedrich, Frédéric Zuhorn, Randolf Klingebiel, Friedrich G. Woermann, Sabine Oertelt-Prigione, and Wolf-Rüdiger Schäbitz. 2022. "Transient Global Amnesia (TGA): Sex-Specific Differences in Blood Pressure and Cerebral Microangiopathy in Patients with TGA" Journal of Clinical Medicine 11, no. 19: 5803. https://doi.org/10.3390/jcm11195803
APA StyleRogalewski, A., Beyer, A., Friedrich, A., Zuhorn, F., Klingebiel, R., Woermann, F. G., Oertelt-Prigione, S., & Schäbitz, W.-R. (2022). Transient Global Amnesia (TGA): Sex-Specific Differences in Blood Pressure and Cerebral Microangiopathy in Patients with TGA. Journal of Clinical Medicine, 11(19), 5803. https://doi.org/10.3390/jcm11195803







