Current Approaches to Osteoid Osteoma and Minimally Invasive Surgery—A Minireview and a Case Report
Abstract
1. Introduction
2. Epidemiology
3. Classification
4. Origin and Pathophysiology
5. Pathology
6. Diagnosis
6.1. Clinical Symptoms
6.2. Imaging Methods
6.3. Biopsy
7. Therapy
7.1. Spontaneous Regression
7.2. Medical Therapy
7.3. Open Surgery
7.4. Minimally Invasive Approaches
8. Differential Diagnosis
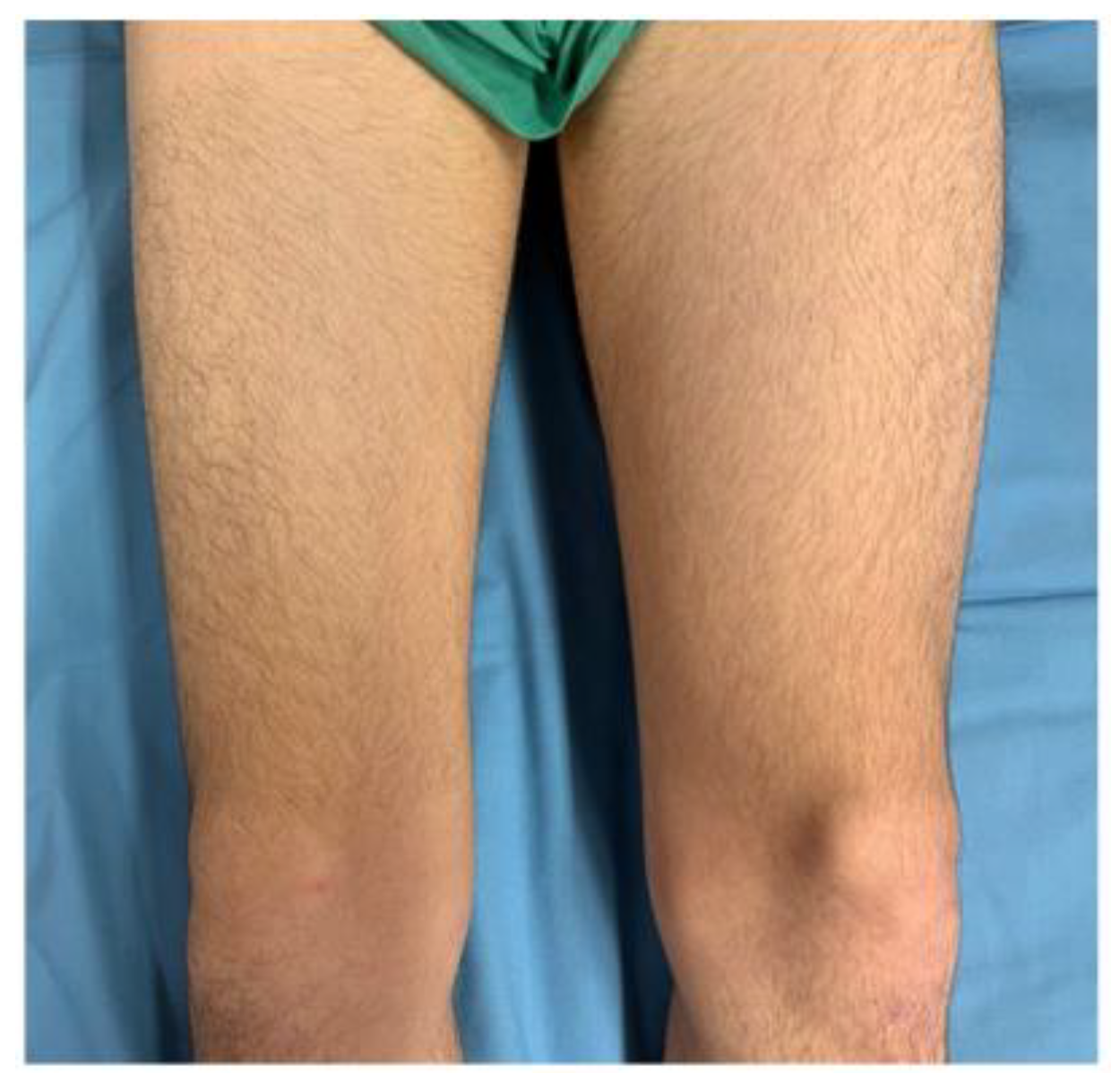
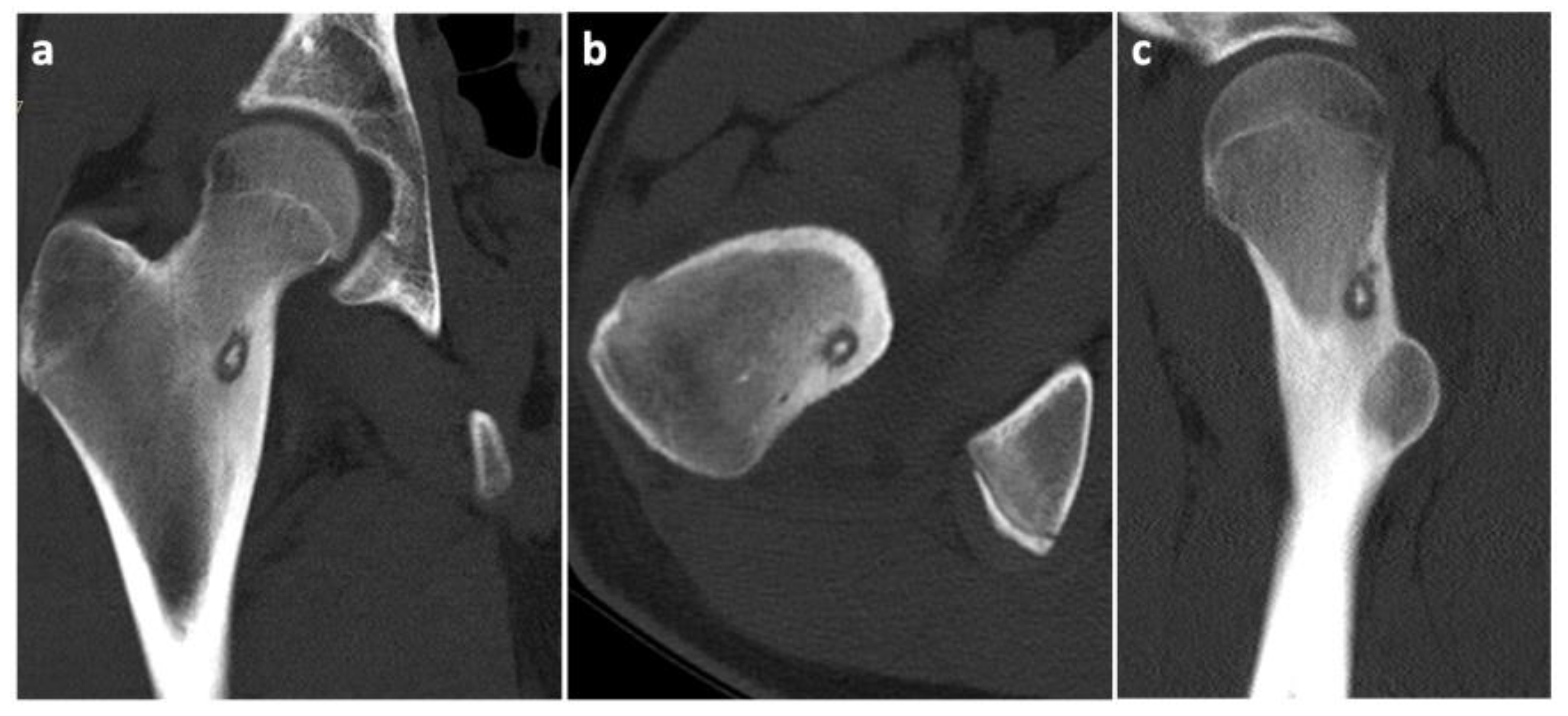
9. Case History
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Rosenberg, A.E. Bone-Forming Tumors. Surg. Pathol. Clin. 2017, 10, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, D.; Learch, T.J.; Ishimitsu, D.N.; Motamedi, K.; Katz, M.D.; Brien, E.W.; Menendez, L. Thermal ablation of osteoid osteoma: Overview and step-by-step guide. Radiographics 2009, 29, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Lindner, N.J.; Ozaki, T.; Roedl, R.; Gosheger, G.; Winkelmann, W.; Wörtler, K. Percutaneous radiofrequency ablation in osteoid osteoma. J. Bone Jt. Surg. Br. 2001, 83, 391–396. [Google Scholar] [CrossRef]
- Tepelenis, K.; Skandalakis, G.P.; Papathanakos, G.; Kefala, M.A.; Kitsouli, A.; Barbouti, A.; Tepelenis, N.; Varvarousis, D.; Vlachos, K.; Kanavaros, P.; et al. Osteoid Osteoma: An Updated Review of Epidemiology, Pathogenesis, Clinical Presentation, Radiological Features, and Treatment Option. In Vivo 2021, 35, 1929–1938. [Google Scholar] [CrossRef]
- De Filippo, M.; Russo, U.; Papapietro, V.R.; Ceccarelli, F.; Pogliacomi, F.; Vaienti, E.; Piccolo, C.; Capasso, R.; Sica, A.; Cioce, F. Radiofrequency ablation of osteoid osteoma. Acta Biomed. 2018, 89, 175–185. [Google Scholar]
- Unni, K.K.; Dahlin, D.C. Osteoid osteoma. In Dahlin’s Bone Tumors, 5th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1996; pp. 121–130. [Google Scholar]
- Boscainos, P.J.; Cousins, G.R.; Kulshreshtha, R.; Oliver, T.B.; Papagelopoulos, P.J. Osteoid osteoma. Orthopedics 2013, 36, 792–800. [Google Scholar] [CrossRef]
- Hakim, D.N.; Pelly, T.; Kulendran, M.; Caris, J.A. Benign tumours of the bone: A review. J. Bone Oncol. 2015, 4, 37–41. [Google Scholar] [CrossRef]
- Sahin, C. Secondary radiological findings of osteoid osteoma as muscular atrophy and synovitis in paediatric and adult patients. Pol. J. Radiol. 2020, 85, 316–322. [Google Scholar] [CrossRef]
- Jackson, R.P.; Reckling, F.W.; Mants, F.A. Osteoid osteoma and osteoblastoma. Similar histologic lesions with different natural histories. Clin. Orthop. Relat. Res. 1977, 128, 303–313. [Google Scholar]
- Themistocleous, G.S.; Chloros, G.D.; Benetos, I.S.; Efstathopoulos, D.G.; Gerostathopoulos, N.E.; Soucacos, P.N. Osteoid osteoma of the upper extremity. A diagnostic challenge. Chir. Main 2006, 25, 69–76. [Google Scholar] [CrossRef]
- Carneiro, B.C.; Da Cruz, I.A.N.; Filho, A.G.O.; Silva, I.P.; Guimarães, J.B.; Silva, F.D.; Nico, M.A.C.; Stump, X.M.G.R.G. Osteoid osteoma: The great mimicker. Insights Imaging 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Chandler, F.A.; Kaell, H.I. Osteoid-osteoma. Arch. Surg. 1950, 60, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Stastka, P.; Jackson, G.; White, M.; Khalefih, S.; Singh, Y.; Lima, A.; Aziz, M. Parosteal osteosarcoma arising from the site of a prior excised osteoid osteoma: A rare progression, or an uncommon coincidence? Report of a case with brief review of the literature. JSM Clin. Cytol. Pathol. 2020, 4, 1–4. [Google Scholar]
- Noordin, S.; Allana, S.; Hilal, K.; Nadeem, N.; Lakdawala, R.; Sadruddin, A.; Uddin, N. Osteoid osteoma: Contemporary management. Orthop. Rev. 2018, 10, 7496. [Google Scholar] [CrossRef] [PubMed]
- Baruffi, M.R.; Volpon, J.B.; Neto, J.B.; Casartelli, C. Osteoid osteomas with chromosome alterations involving 22q. Cancer Genet. Cytogenet. 2001, 124, 127–131. [Google Scholar] [CrossRef]
- Lee, E.H.; Shafi, M.; Hui, J.H. Osteoid osteoma: A current review. J. Pediatr. Orthop. 2006, 26, 695–700. [Google Scholar] [CrossRef]
- Zileli, M.; Cagli, S.; Basdemir, G.; Ersahin, Y. Osteoid osteomas and osteoblastomas of the spine. Neurosurg. Focus 2003, 15, 1–7. [Google Scholar] [CrossRef]
- Frassica, F.J.; Waltrip, R.L.; Sponseller, P.D.; Ma, L.D.; McCarthy, E.F., Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop. Clin. N. Am. 1996, 27, 559–574. [Google Scholar] [CrossRef]
- Greco, F.; Tamburrelli, F.; Ciabattoni, G. Prostaglandins in osteoid osteoma. Int. Orthop. 1991, 15, 35–37. [Google Scholar] [CrossRef]
- Kitsoulis, P.; Mantellos, G.; Vlychou, M. Osteoid osteoma. Acta Orthop. Belg. 2006, 72, 119–125. [Google Scholar]
- Ozaki, T.; Liljenqvist, U.; Hillmann, A.; Halm, H.; Lindner, N.; Gosheger, G.; Winkelmann, W. Osteoid osteoma and osteoblastoma of the spine: Experiences with 22 patients. Clin. Orthop. Relat. Res. 2002, 397, 394–402. [Google Scholar] [CrossRef] [PubMed]
- García-Vivar, M.L.; Galindez, E.; Aróstegui, J.; García Llorente, F.; Uriarte, E.; Aramburu, J.M. Monoartritis crónica por osteoma osteoide [Chronic monoarthritis caused by osteoid osteoma]. An. De Med. Interna 1996, 13, 344–346. [Google Scholar]
- Houdek, M.T.; Wenger, D.E.; Sherman, C.E.; Turner, N.S. Osteoid osteomas of the foot and ankle: A study of patients over a 20-year period. Am. J. Orthop. 2014, 43, 552–556. [Google Scholar]
- Harish, S.; Saifuddin, A. Imaging features of spinal osteoid osteoma with emphasis on MRI findings. Eur. Radiol. 2005, 15, 2396–2403. [Google Scholar] [CrossRef]
- Norman, A.; Dorfman, H.D. Osteoid-osteoma inducing pronounced overgrowth and deformity of bone. Clin. Orthop. Relat. Res. 1975, 110, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Spouge, A.R.; Thain, L.M. Osteoid osteoma: MR imaging revisited. Clin. Imaging 2000, 24, 19–27. [Google Scholar] [CrossRef]
- Gassert, F.; Hammel, J.; Hofmann, F.; Neumann, J.; von Schacky, C.; Gassert, F.; Pfeiffer, D.; Pfeiffer, F.; Makowski, M.; Woertler, K.; et al. Detection of Bone Marrow Edema in Patients with Osteoid Osteoma Using Three-Material Decomposition with Dual-Layer Spectral CT. Diagnostics 2021, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, A.; Martella, C.; Ceccarelli, L.; Miceli, M.; Spinnato, P.; Facchini, G. Osteoid osteoma: Which is the best mininvasive treatment option? Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1611–1624. [Google Scholar] [CrossRef]
- Aiba, H.; Hayashi, K.; Inatani, H.; Satoshi, Y.; Watanabe, N.; Sakurai, H.; Tsuchiya, H.; Otsuka, T. Conservative treatment for patients with osteoid osteoma: A case series. Anticancer Res. 2014, 34, 3721–3725. [Google Scholar]
- Carpintero-Benitez, P.; Aguirre, M.A.; Serrano, J.A.; Lluch, M. Effect of rofecoxib on pain caused by osteoid osteoma. Orthopedics 2004, 27, 1188–1191. [Google Scholar] [CrossRef]
- Kirchner, B.; Hillmann, A.; Lottes, G.; Sciuk, J.; Bartenstein, P.; Winkelmann, W.; Schober, O. Intraoperative, probe-guided curettage of osteoid osteoma. Eur. J. Nucl. Med. 1993, 20, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Pettine, K.A.; Klassen, R.A. Osteoid-osteoma and osteoblastoma of the spine. J. Bone Joint Surg. Am. 1986, 68, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.H.; Ghelman, B. Osteoid osteoma and osteoblastoma. Current concepts and recent advances. Clin. Orthop. Relat. Res. 1986, 204, 76–85. [Google Scholar] [CrossRef]
- Goto, T.; Shinoda, Y.; Okuma, T.; Ogura, K.; Tsuda, Y.; Yamakawa, K.; Hozumi, T. Administration of nonsteroidal anti-inflammatory drugs accelerates spontaneous healing of osteoid osteoma. Arch. Orthop. Trauma Surg. 2011, 131, 619–625. [Google Scholar] [CrossRef]
- Bousson, V.; Leturcq, T.; Ea, H.K.; Hauger, O.; Mehsen-Cetre, N.; Hamzé, B.; Parlier-Cuau, C.; Laredo, J.-D.; Schaeverbeke, T.; Orcel, P. An open-label, prospective, observational study of the efficacy of bisphosphonate therapy for painful osteoid osteoma. Eur. Radiol. 2018, 28, 478–486. [Google Scholar] [CrossRef]
- Yu, F.; Niu, X.H.; Zhang, Q.; Zhao, H.T.; Xu, L.H.; Deng, Z.P. Radiofrequency ablation under 3D intraoperative Iso-C C-arm navigation for the treatment of osteoid osteomas. Br. J. Radiol. 2015, 88, 20140535. [Google Scholar] [CrossRef]
- Oc, Y.; Kilinc, B.E.; Cennet, S.; Boyacioglu, M.M.; Ertugrul, R.; Varol, A. Complications of Computer Tomography Assisted Radiofrequency Ablation in the Treatment of Osteoid Osteoma. Biomed. Res. Int. 2019, 2019, 4376851. [Google Scholar] [CrossRef]
- Pipola, V.; Tedesco, G.; Spinnato, P.; Facchini, G.; Gala, R.B.; Bandiera, S.; Bròdano, G.B.; Terzi, S.; Ghermandi, R.; Evangelisti, G.; et al. Surgery Versus Radiofrequency Ablation in the Management of Spinal Osteoid Osteomas: A Spine Oncology Referral Center Comparison Analysis of 138 Cases. World Neurosurg. 2021, 145, 298–304. [Google Scholar] [CrossRef]
- Le Corroller, T.; Vives, T.; Mattei, J.C.; Pauly, V.; Guenoun, D.; Rochwerger, A.; Champsaur, P. Osteoid Osteoma: Percutaneous CT-guided Cryoablation Is a Safe, Effective, and Durable Treatment Option in Adults. Radiology 2022, 302, 392–399. [Google Scholar] [CrossRef]
- Coupal, T.M.; Mallinson, P.I.; Munk, P.L.; Liu, D.; Clarkson, P.; Ouellette, H. CT-guided percutaneous cryoablation for osteoid osteoma: Initial experience in adults. Am. J. Roentgenol. 2014, 202, 1136–1139. [Google Scholar] [CrossRef]
- Roqueplan, F.; Porcher, R.; Hamzé, B.; Bousson, V.; Zouari, L.; Younan, T.; Parlier-Cuau, C.; Laredo, J.-D. Long-term results of percutaneous resection and interstitial laser ablation of osteoid osteomas. Eur. Radiol. 2010, 20, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.D.; Hall-Craggs, M.A.; Ripley, P.; Cobb, J.P.; Bown, S.G. Interstitial laser photocoagulation for the treatment of osteoid osteoma. J. Bone Jt. Surg. Br. 2000, 82, 1125–1128. [Google Scholar] [CrossRef]
- Napoli, A.; Bazzocchi, A.; Scipione, R.; Anzidei, M.; Saba, L.; Ghanouni, P.; Cozzi, D.A.; Catalano, C. Noninvasive Therapy for Osteoid Osteoma: A Prospective Developmental Study with MR Imaging-guided High-Intensity Focused Ultrasound. Radiology 2017, 285, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Tempany, C.M.; McDannold, N.J.; Hynynen, K.; Jolesz, F.A. Focused ultrasound surgery in oncology: Overview and principles. Radiology 2011, 259, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Hopkins, T.; Morris, J.; Tran, N.D.; David, E.; Massari, F.; Farid, H.; Vogel, A.; O’Connell, W.G.; Sunenshine, P.; et al. Radiofrequency Ablation for the Palliative Treatment of Bone Metastases: Outcomes from the Multicenter OsteoCool Tumor Ablation Post-Market Study (OPuS One Study) in 100 Patients. J. Vasc. Interv. Radiol. 2020, 31, 1745–1752. [Google Scholar] [CrossRef]
- Lin, N.; Ye, Z.-M.; Qu, H.; Yan, X.-B.; Pan, W.-B.; Huang, X.; Liu, M. Open Surgery for Osteoid Osteoma with Three Dimensional C-arm Scan under the Guidance of Computer Navigation. Orthop. Surg. 2016, 8, 205–211. [Google Scholar] [CrossRef]
- Yuce, G.; Aytekin, N.; Eren, S.; Genç, B.; Ateş, Ö.F.; Canyiğit, M. Is radiofrequency ablation safe and effective in treating osteoid osteomas? A prospective single-center study with atypical cases. J. Orthop. Surg. 2020, 28, 1. [Google Scholar] [CrossRef]
- Yang, W.T.; Chen, W.M.; Wang, N.H.; Chen, T.H. Surgical treatment for osteoid osteoma—Experience in both conventional open excision and CT-guided mini-incision surgery. J. Chin. Med. Assoc. 2007, 70, 545–550. [Google Scholar] [CrossRef]
- Hamdi, M.F.; Tarhouni, L.; Daghfous, M.; Bergaoui, N.; Baccari, S. Osteoid osteoma of the phalanx and metacarpal bone: Report of 17 cases. Musculoskelet Surg. 2015, 99, 61–65. [Google Scholar] [CrossRef]
- Vanderschueren, G.M.; Obermann, W.R.; Dijkstra, S.P.; Taminiau, A.H.; Bloem, J.L.; van Erkel, A.R. Radiofrequency ablation of spinal osteoid osteoma: Clinical outcome. Spine 2009, 34, 901–904. [Google Scholar] [CrossRef]
- Weber, M.-A.; Sprengel, S.D.; Omlor, G.W.; Lehner, B.; Wiedenhöfer, B.; Kauczor, H.-U.; Rehnitz, C. Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal osteoid osteomas in comparison to open surgical resection. Skelet. Radiol. 2015, 44, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Hornicek, F.J.; Wolfe, M.W.; Jennings, L.C.; Gebhardt, M.C.; Mankin, H.J. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J. Bone Jt. Surg. Am. 1998, 80, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Mallepally, A.R.; Mahajan, R.; Pacha, S.; Rustagi, T.; Marathe, N.; Chhabra, H.S. Spinal osteoid osteoma: Surgical resection and review of literature. Surg. Neurol. Int. 2020, 11, 308. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerny, J.; Soukup, J.; Cerna, S.; Novotny, T. Current Approaches to Osteoid Osteoma and Minimally Invasive Surgery—A Minireview and a Case Report. J. Clin. Med. 2022, 11, 5806. https://doi.org/10.3390/jcm11195806
Cerny J, Soukup J, Cerna S, Novotny T. Current Approaches to Osteoid Osteoma and Minimally Invasive Surgery—A Minireview and a Case Report. Journal of Clinical Medicine. 2022; 11(19):5806. https://doi.org/10.3390/jcm11195806
Chicago/Turabian StyleCerny, Jan, Jan Soukup, Sarka Cerna, and Tomas Novotny. 2022. "Current Approaches to Osteoid Osteoma and Minimally Invasive Surgery—A Minireview and a Case Report" Journal of Clinical Medicine 11, no. 19: 5806. https://doi.org/10.3390/jcm11195806
APA StyleCerny, J., Soukup, J., Cerna, S., & Novotny, T. (2022). Current Approaches to Osteoid Osteoma and Minimally Invasive Surgery—A Minireview and a Case Report. Journal of Clinical Medicine, 11(19), 5806. https://doi.org/10.3390/jcm11195806






