Novel Surgical Technique for Adolescent Idiopathic Scoliosis: Minimally Invasive Scoliosis Surgery
Abstract
:1. Introduction
2. Considerations in Determining MISS or COSS
3. Surgical Techniques
3.1. Former Technique
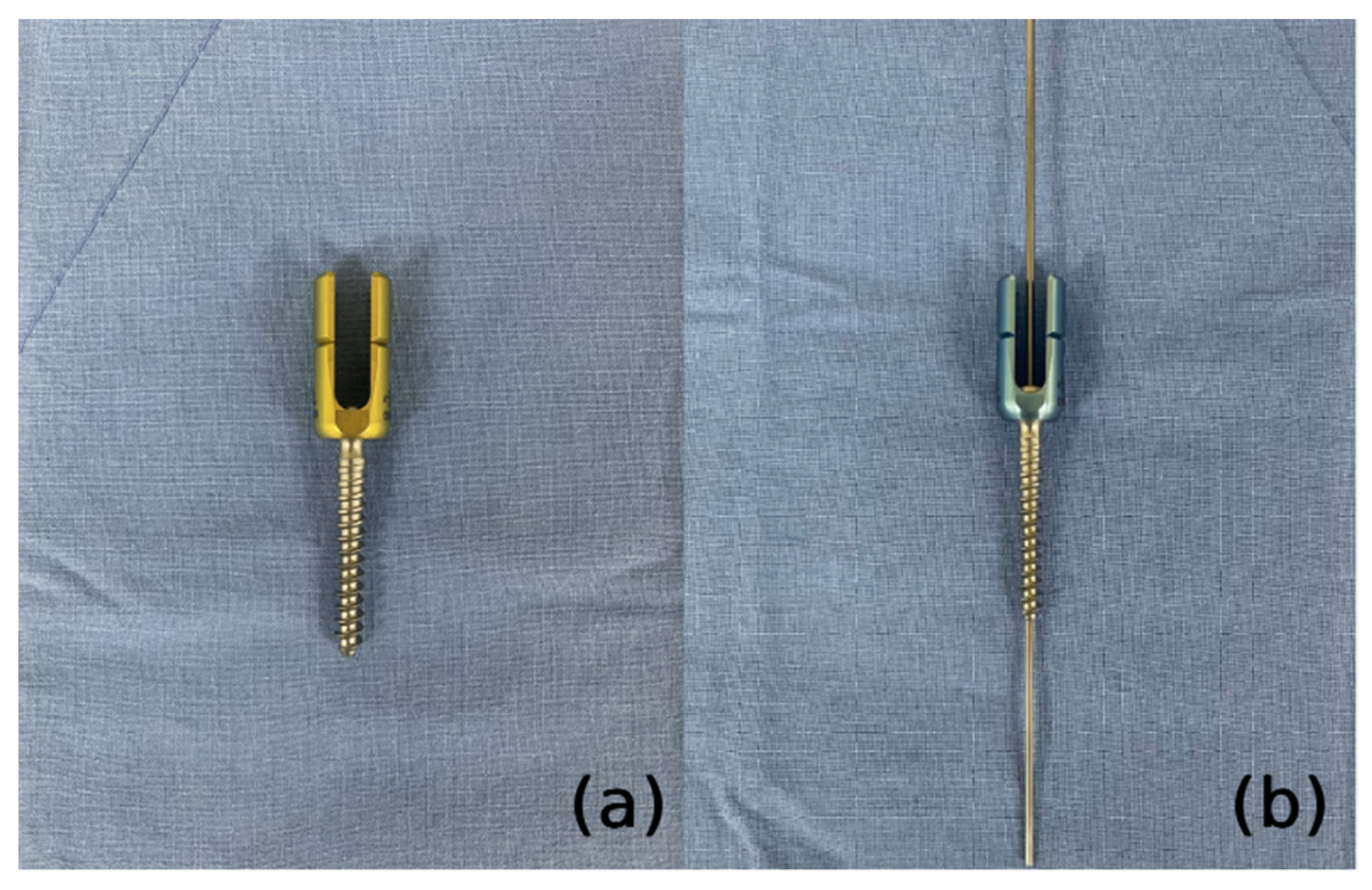
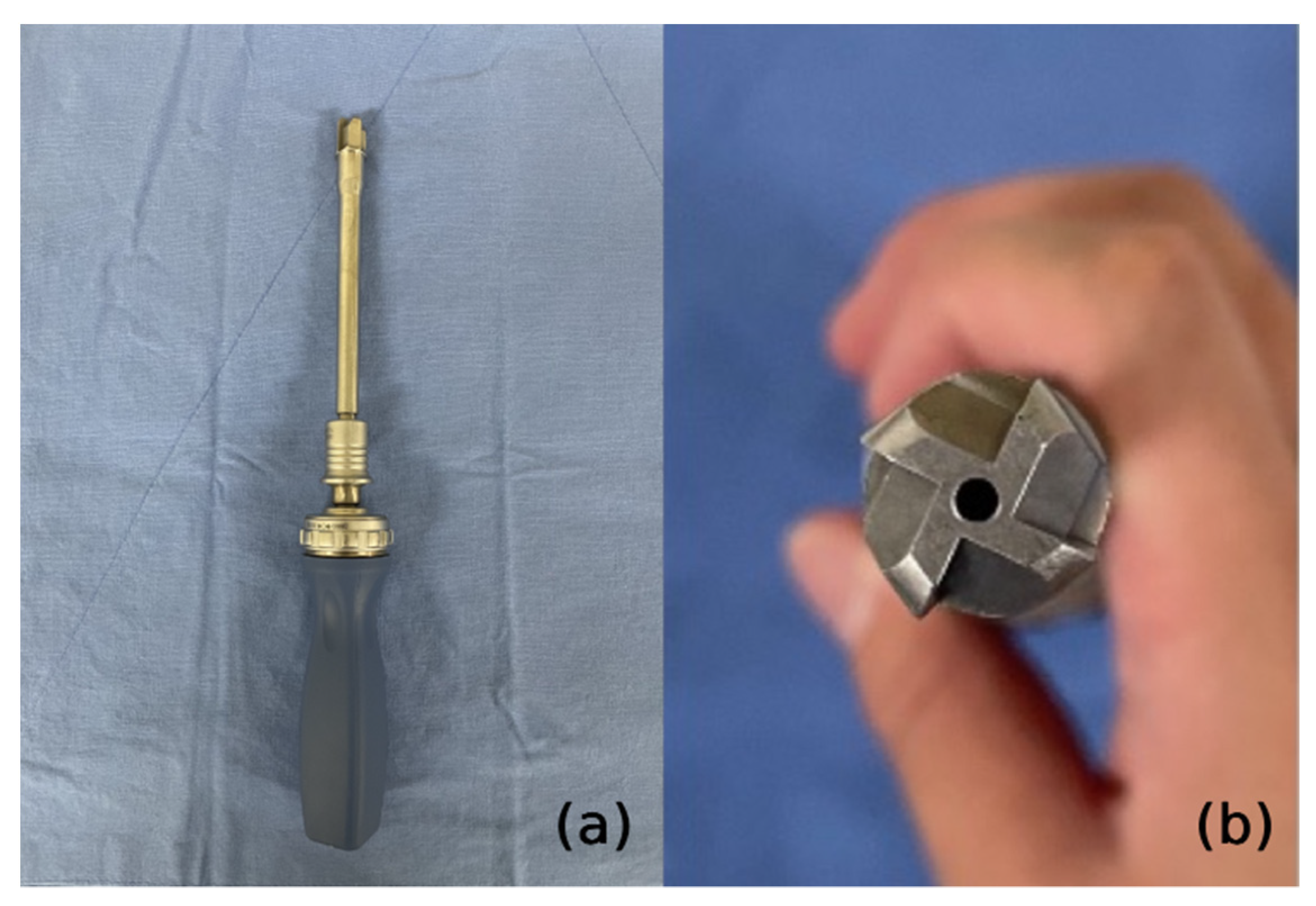
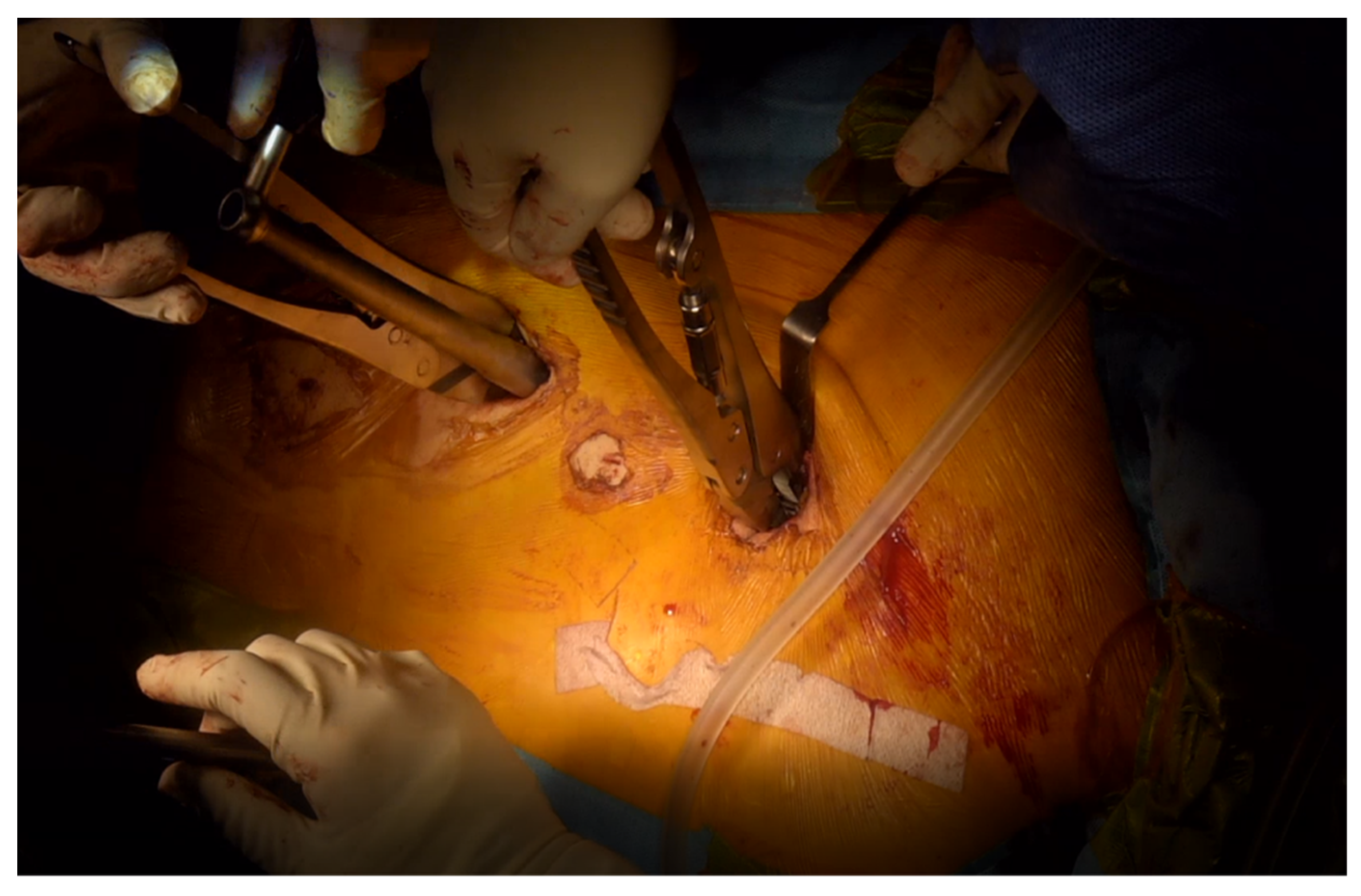
3.2. Our Group’s Technique
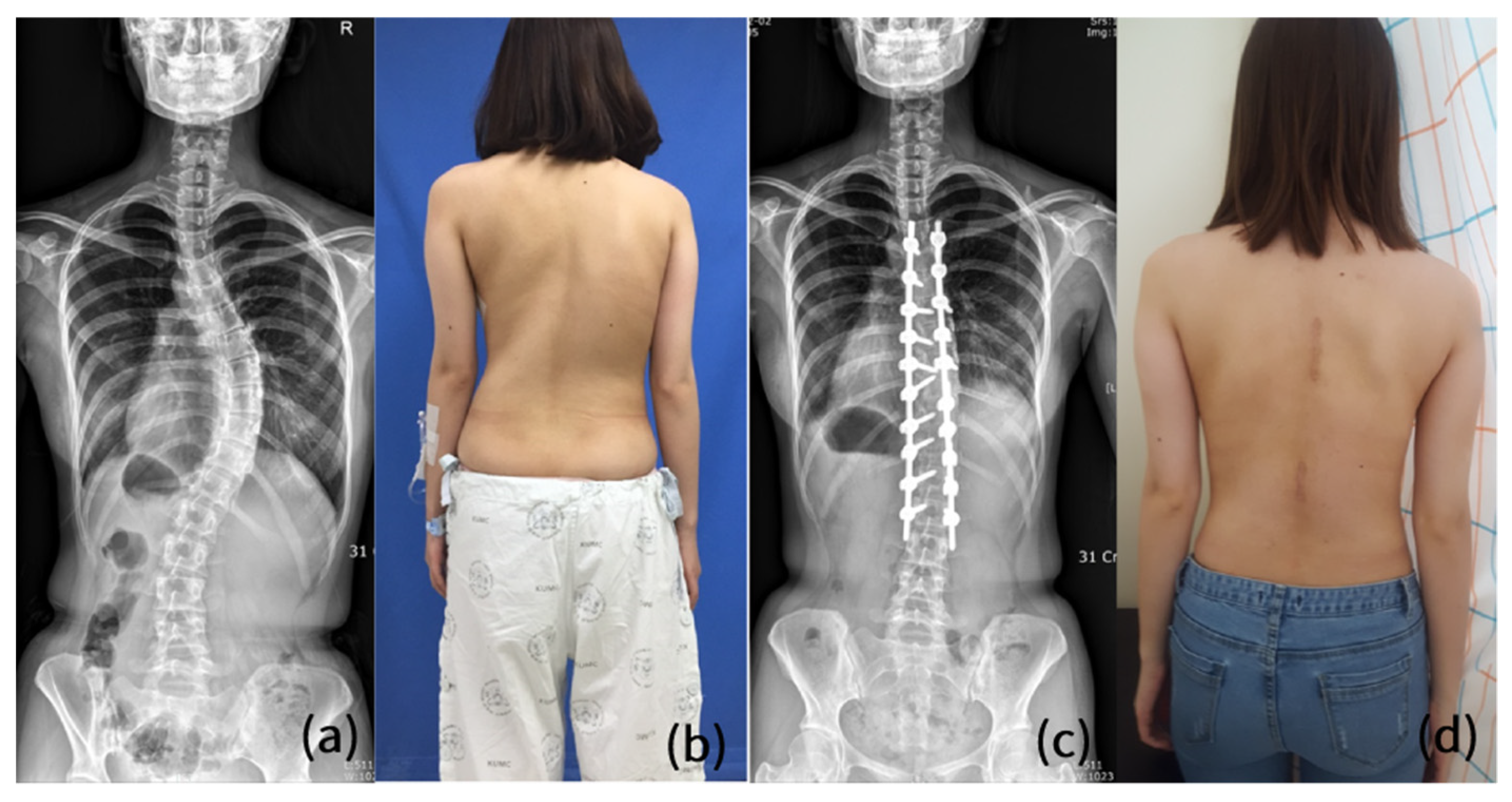
4. Comparisons with Conventional Open Scoliosis Surgery Regarding Surgical Outcome
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, P.D.; Canseco, J.A.; Houlihan, N.; Gabay, A.; Grasso, G.; Vaccaro, A.R. Overview of minimally invasive spine surgery. World Neurosurg. 2020, 142, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ham, D.-W.; Kwon, B.-T.; Park, S.-M.; Kim, H.-J.; Yeom, J.S. Minimally invasive spine surgery: Techniques, technologies, and indications. Asian Spine J. 2020, 14, 694. [Google Scholar] [CrossRef] [PubMed]
- Riseborough, E.J.; Wynne-Davies, R. A Genetic Survey of Idiopathic Scoliosis in Boston, Massachusetts. J. Bone Jt. Surg. 1973, 55, 974–982. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, H.J.; Chang, D.-G.; Suh, S.W. Comparative Analysis of Radiologic and Clinical Outcomes between Conventional Open and Minimally Invasive Scoliosis Surgery for Adolescent Idiopathic Scoliosis. World Neurosurg. 2021, 151, e234–e240. [Google Scholar] [CrossRef]
- Sarwahi, V.; Horn, J.J.; Kulkarni, P.M.; Wollowick, A.L.; Lo, Y.; Gambassi, M.; Amaral, T.D. Minimally invasive surgery in patients with adolescent idiopathic scoliosis: Is it better than the standard approach? A 2-year follow-up study. Clin. Spine Surg. 2016, 29, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Suk, S.-I.; Chung, E.-R. Direct vertebral rotation: A new technique of three-dimensional deformity correction with segmental pedicle screw fixation in adolescent idiopathic scoliosis. Spine 2004, 29, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Sarwahi, V.; Wollowick, A.L.; Sugarman, E.P.; Horn, J.J.; Gambassi, M.; Amaral, T.D. Minimally invasive scoliosis surgery: An innovative technique in patients with adolescent idiopathic scoliosis. Scoliosis 2011, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyanji, F.; Desai, S. Minimally invasive surgical options for adolescent idiopathic scoliosis. Semin. Spine Surg. 2015, 27, 39–44. [Google Scholar] [CrossRef]
- Brodano, G.B.; Martikos, K.; Vommaro, F.; Greggi, T.; Boriani, S. Less invasive surgery in idiopathic scoliosis: A case report. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 24–28. [Google Scholar]
- Yang, J.H.; Chang, D.-G.; Suh, S.W.; Damani, N.; Lee, H.-N.; Lim, J.; Mun, F. Safety and effectiveness of minimally invasive scoliosis surgery for adolescent idiopathic scoliosis: A retrospective case series of 84 patients. Eur. Spine J. 2020, 29, 761–769. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, H.J.; Chang, D.-G.; Suh, S.W. Minimally invasive scoliosis surgery for adolescent idiopathic scoliosis using posterior mini-open technique. J. Clin. Neurosci. 2021, 89, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Q.; Wang, C.-F.; Bai, Y.-S.; Zhu, X.-D.; Yang, C.-W.; Xie, Y.; Li, M. Using precisely controlled bidirectional orthopedic forces to assess flexibility in adolescent idiopathic scoliosis: Comparisons between push-traction film, supine side bending, suspension, and fulcrum bending film. Spine 2011, 36, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Klepps, S.J.; Lenke, L.G.; Bridwell, K.H.; Bassett, G.S.; Whorton, J. Prospective comparison of flexibility radiographs in adolescent idiopathic scoliosis. Spine 2001, 26, E74–E79. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Chang, D.-G.; Honnurappa, A.R.; Kim, S.-H.; Ham, C.-H.; Suh, S.-W. Minimally invasive surgery for correcting adolescent idiopathic scoliosis: A novel approach called coin hole technique. J. Adv. Spine Surg. 2016, 6, 20–28. [Google Scholar]
- Weinstein, S.L.; Dolan, L.A.; Cheng, J.C.; Danielsson, A.; Morcuende, J.A. Adolescent idiopathic scoliosis. Lancet 2008, 371, 1527–1537. [Google Scholar] [CrossRef] [Green Version]
- Suk, S.-I.; Kim, J.-H.; Kim, S.-S.; Lim, D.-J. Pedicle screw instrumentation in adolescent idiopathic scoliosis (AIS). Eur. Spine J. 2012, 21, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Théroux, J.; Stomski, N.; Innes, S.; Ballard, A.; Khadra, C.; Labelle, H.; Le May, S. Revisiting the psychometric properties of the Scoliosis Research Society-22 (SRS-22) French version. Scoliosis Spinal Disord. 2017, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Miladi, L.; Gaume, M.; Khouri, N.; Johnson, M.; Topouchian, V.; Glorion, C. Minimally invasive surgery for neuromuscular scoliosis: Results and complications in a series of one hundred patients. Spine 2018, 43, E968. [Google Scholar] [CrossRef]
- Gaume, M.; Vergari, C.; Khouri, N.; Skalli, W.; Glorion, C.; Miladi, L. Minimally invasive surgery for neuromuscular scoliosis: Results and complications at a minimal follow-up of 5 years. Spine 2021, 46, 1696–1704. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Liang, Z.; Zhou, M.; Chen, C. Comparative clinical effectiveness and safety of bone morphogenetic protein versus autologous iliac crest bone graft in lumbar fusion: A meta-analysis and systematic review. Spine 2020, 45, E729. [Google Scholar] [CrossRef]
- Mariscal, G.; Nuñez, J.H.; Barrios, C.; Domenech-Fernandez, P. A meta-analysis of bone morphogenetic protein-2 versus iliac crest bone graft for the posterolateral fusion of the lumbar spine. J. Bone Miner. Metab. 2020, 38, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Fujibayashi, S.; Otsuki, B.; Shimizu, T.; Matsuda, S. Repair of iliac crest defects with a hydroxyapatite/collagen composite. Asian Spine J. 2020, 14, 808. [Google Scholar] [CrossRef] [PubMed]
- Reintjes, S.L.; Amankwah, E.K.; Rodriguez, L.F.; Carey, C.C.; Tuite, G.F. Allograft versus autograft for pediatric posterior cervical and occipito-cervical fusion: A systematic review of factors affecting fusion rates. J. Neurosurg. Pediatr. 2016, 17, 187–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfiky, T.A.; Patil, N.D.; Allam, Y.; Ragab, R. Endplate changes with Polyetheretherketone cages in posterior lumbar Interbody fusion. Asian Spine J. 2020, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Choi, S.H.; Lee, M.K.; Kang, C.-N. Efficacy and safety of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in additional lumbar posterolateral fusion: Minimum 1-year follow-up. Spine J. 2021, 21, 1340–1346. [Google Scholar] [CrossRef]
- Rocque, B.G.; Kelly, M.P.; Miller, J.H.; Li, Y.; Anderson, P.A. Bone morphogenetic protein–associated complications in pediatric spinal fusion in the early postoperative period: An analysis of 4658 patients and review of the literature. J. Neurosurg. Pediatr. 2014, 14, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Sayama, C.; Hadley, C.; Monaco, G.N.; Sen, A.; Brayton, A.; Briceño, V.; Tran, B.H.; Ryan, S.L.; Luerssen, T.G.; Fulkerson, D. The efficacy of routine use of recombinant human bone morphogenetic protein–2 in occipitocervical and atlantoaxial fusions of the pediatric spine: A minimum of 12 months’ follow-up with computed tomography. J. Neurosurg. Pediatr. 2015, 16, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Kebaish, K.M.; Sponseller, P.D. Factors Associated with Use of Bone Morphogenetic Protein During Pediatric Spinal Fusion Surgery. J. Bone Jt. Surg. 2013, 95, 1265–1270. [Google Scholar] [CrossRef]
- Stiel, N.; Hissnauer, T.N.; Rupprecht, M.; Babin, K.; Schlickewei, C.W.; Rueger, J.M.; Stuecker, R.; Spiro, A.S. Evaluation of complications associated with off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in pediatric orthopaedics. J. Mater. Sci. Mater. Med. 2016, 27, 184. [Google Scholar] [CrossRef]
- Abd-El-Barr, M.M.; Cox, J.B.; Antonucci, M.U.; Bennett, J.; Murad, G.J.; Pincus, D.W. Recombinant human bone morphogenetic protein-2 as an adjunct for spine fusion in a pediatric population. Pediatr. Neurosurg. 2011, 47, 266–271. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.-P.; Mahesh, M.; Gould, R.; De González, A.B.; Miglioretti, D.L. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Elliston, C.D.; Hall, E.J.; Berdon, W.E. Estimated risks of radiation-induced fatal cancer from pediatric CT. Am. J. Roentgenol. 2001, 176, 289–296. [Google Scholar] [CrossRef] [PubMed]

| Values | |
|---|---|
| Total number of patients | 52 |
| Age | 15.31 ± 2.03 * |
| Sex (n): men/women | 2/50 |
| Body mass index (kg/m2) | 19.27 ± 2.79 * |
| Lenke classification (n): 1/2/3/4/5/6 | 39/4/6/0/2/1 |
| King classification (n): 1/2/3/4/5 | 7/11/26/5/3 |
| Duration of surgery (min) | 390.00 ± 91.69 * |
| Estimated blood loss (mL) | 1135.96 ± 678.20 * |
| Number of levels operated (n) | 10.88 ± 1.37 * |
| Radiological parameters | |
| Preoperative Cobb angle (°) | 62.00 ± 9.75 * |
| Cobb angle in bending films (°) | 46.13 ± 14.33 * |
| Flexibility (%) | 26.86 ± 15.75 * |
| Postoperative Cobb angle (°) | 22.40 ± 7.03 * |
| ∆ Cobb angle (°) | 38.02 ± 8.70 * |
| Correction rate (%) | 64.02 ± 8.96 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.C.; Son, S.W.; Yang, J.H.; Chang, D.-G.; Suh, S.W.; Nam, Y.; Kim, H.J. Novel Surgical Technique for Adolescent Idiopathic Scoliosis: Minimally Invasive Scoliosis Surgery. J. Clin. Med. 2022, 11, 5847. https://doi.org/10.3390/jcm11195847
Park SC, Son SW, Yang JH, Chang D-G, Suh SW, Nam Y, Kim HJ. Novel Surgical Technique for Adolescent Idiopathic Scoliosis: Minimally Invasive Scoliosis Surgery. Journal of Clinical Medicine. 2022; 11(19):5847. https://doi.org/10.3390/jcm11195847
Chicago/Turabian StylePark, Sung Cheol, Sei Wook Son, Jae Hyuk Yang, Dong-Gune Chang, Seung Woo Suh, Yunjin Nam, and Hong Jin Kim. 2022. "Novel Surgical Technique for Adolescent Idiopathic Scoliosis: Minimally Invasive Scoliosis Surgery" Journal of Clinical Medicine 11, no. 19: 5847. https://doi.org/10.3390/jcm11195847





