A Rare Case of Tricuspid Valve Libman–Sacks Endocarditis in a Pregnant Woman with Primary Antiphospholipid Syndrome
Abstract
:1. Introduction
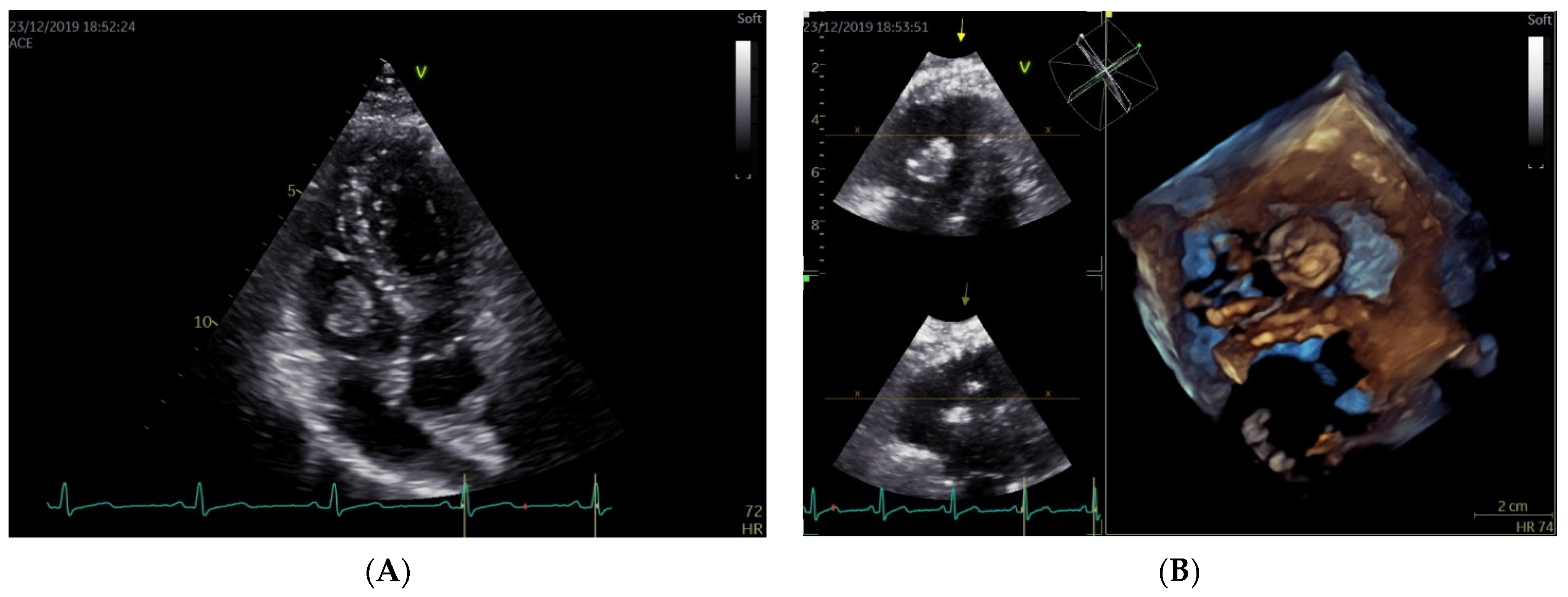
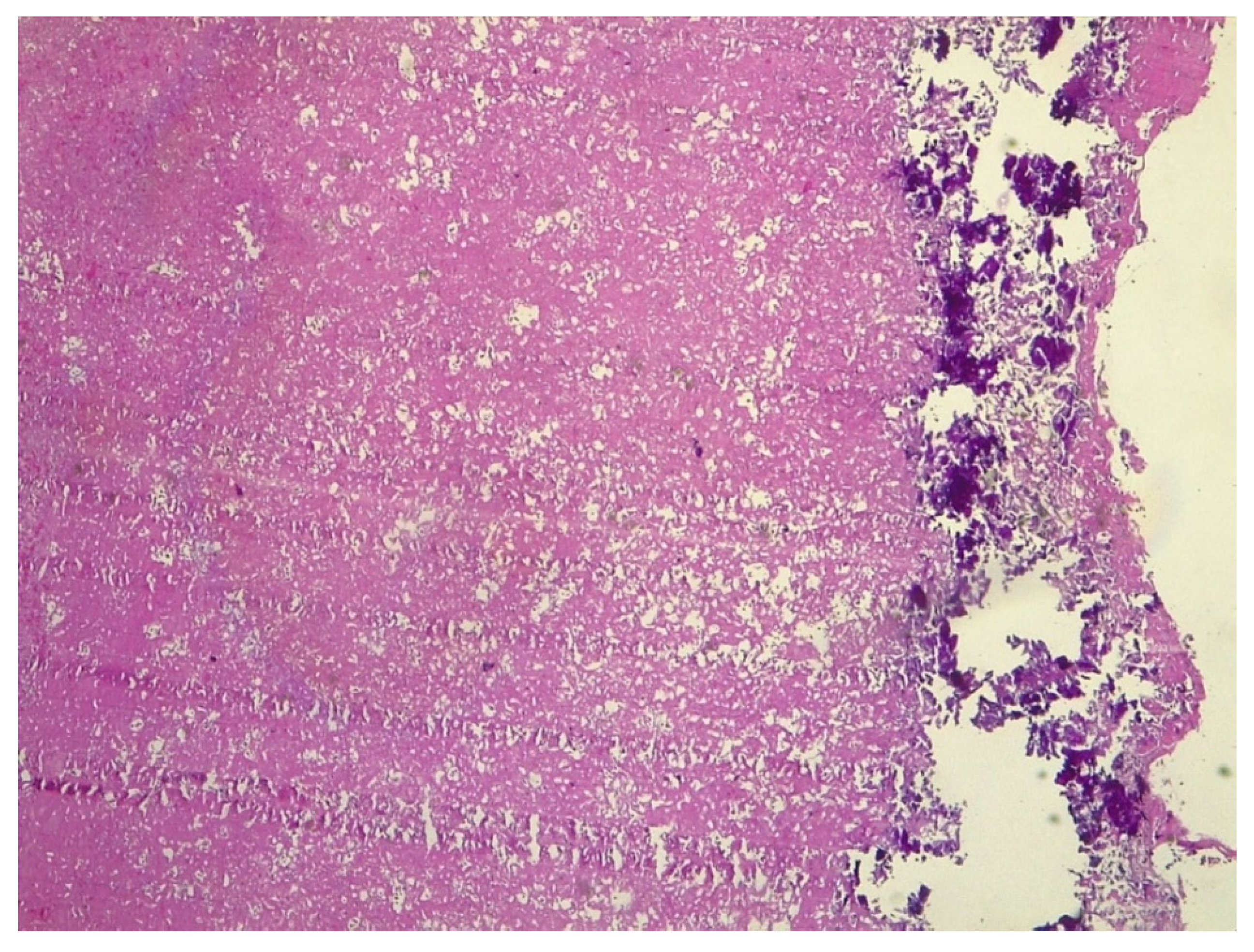
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, B.-W.; Lee, S.-W.; Song, J.J.; Park, Y.-B.; Jung, S.M. Clinical characteristics and long-term outcomes of Libman–Sacks endocarditis in patients with systemic lupus erythematosus. Lupus 2020, 29, 1115–1120. [Google Scholar] [CrossRef]
- Gómez-Puerta, J.A.; Cervera, R. Diagnosis and classification of the antiphospholipid syndrome. J. Autoimmun. 2014, 48–49, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Branch, D.; Rauch, J. The antiphospholipid syndrome. N. Engl. J. Med. 2002, 346, 752–763. [Google Scholar] [CrossRef] [Green Version]
- George, D.; Erkan, D. Antiphospholipid syndrome. Prog. Cardiovasc. Dis. 2009, 52, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R. Antiphospholipid syndrome. Thromb. Res. 2017, 151 (Suppl. 1), S43–S47. [Google Scholar] [CrossRef]
- Kaul, M.; Erkan, D.; Sammaritano, L.; Lockshin, M.D. Assessment of the 2006 revised antiphospholipid syndrome classification criteria. Ann. Rheum. Dis. 2007, 66, 927–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Moutsopoulos, H.; Vlachoyiannopoulos, P. Antiphospholipid antibody syndrome. In Harrison’s Principles of International Medicine, 18th ed.; Longo, D., Fauci, A., Kasper, D., Hauser, S., Jameson, J., Loscalzo, J., Eds.; McGraw-Hill Education/Medical: New York, NY, USA, 2011; pp. 2736–2738. [Google Scholar]
- Mattuizzi, A.; Madar, H.; Froeliger, A.; Houssin, C.; Chabanier, P.; Merlot, B.; Lazaro, E.; Elleboode, B.; Sentilhes, L. Complications obstétricales du lupus érythémateux systémique et du SAPL: Une prise en charge multidisciplinaire. Gynécologie Obs. Fertil. Sénologie 2020, 48, 448–452. [Google Scholar] [CrossRef]
- Radin, M.; Cecchi, I.; Schreiber, K.; Rubini, E.; Roccatello, D.; Cuadrado, M.; Sciascia, S. Pregnancy success rate and response to heparins and/or aspirin differ in women with antiphospholipid antibodies according to their Global AntiphosPholipid Syndrome Score. Semin. Arthritis Rheum. 2020, 50, 553–556. [Google Scholar] [CrossRef]
- Whalen, H.; Dako, F.; Patel, P.; Sahbaz, J.; Hong-Zohlman, S.; White, C.S.; Jeudy, J. Role of Imaging for Suspected Cardiac Thrombus. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 81. [Google Scholar] [CrossRef]
- Rose, P.S.; Punjabi, N.M.; Pearse, D.B. Treatment of Right Heart Thromboemboli. Chest 2002, 121, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bailén, M.; López-Caler, C.; Castillo-Rivera, A.; Rucabado-Aguilar, L.; Cuadra, J.R.; Toral, J.L.; Cabezas, C.L.; Guerrero, J.C.F. Giant right atrial thrombi treated with thrombolysis. Can. J. Cardiol. 2008, 24, 312–314. [Google Scholar] [CrossRef]
- Pilato, E.; Pinna, G.B.; Grande, L.; Cirillo, V.; Izzo, R.; Tufano, A.; Guida, M.; Sarno, L.; Browning, R.; Comentale, G. Challenging report of cardiopulmonary bypass in 16th week pregnant patient with endoventricular mass. Heart Lung 2021, 50, 174–176. [Google Scholar] [CrossRef]
- Hojnik, M.; George, J.; Ziporen, L.; Shoenfeld, Y. Heart Valve Involvement (Libman-Sacks Endocarditis) in the Antiphospholipid Syndrome. Circulation 1996, 93, 1579–1587. [Google Scholar] [CrossRef]
- Moyssakis, I.; Tektonidou, M.; Vasilliou, V.A.; Samarkos, M.; Votteas, V.; Moutsopoulos, H.M. Libman-Sacks Endocarditis in Systemic Lupus Erythematosus: Prevalence, Associations, and Evolution. Am. J. Med. 2007, 120, 636–642. [Google Scholar] [CrossRef]
- Moaref, A.; Afifi, S.; Rezaian, S.; Rezaian, G.R. Isolated Tricuspid Valve Libman-Sacks Endocarditis and Valvular Stenosis: Unusual Manifestations of Systemic Lupus Erythematosus. J. Am. Soc. Echocardiogr. 2010, 23, 341.e3–341.e5. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, K.; Negi, P.; Merwaha, R.; Sharma, M. Isolated tricuspid valve Libman-Sacks endocarditis in a patient with antiphospholipid antibody syndrome. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Waldoch, A.; Kwiatkowska, J.; Dorniak, K. Unusual location of the Libman–Sacks endocarditis in a teenager: A case report. Cardiol. Young 2016, 26, 365–367. [Google Scholar] [CrossRef]
- Unnikrishnan, D.; Shaikh, N.; Sharayah, A.; Patton, C. A case of tricuspid valve non-bacterial thrombotic endocarditis presenting as pulmonary embolism in a patient with antiphospholipid antibody syndrome. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Zurick, A.O., III; Yang, E.H.; Chang, P.P.; Hinderliter, A.L.; Willis, P.W., IV. A 38-Year-Old Hispanic Woman with Paradoxical Embolism and Libman-Sacks Endocarditis Involving the Tricuspid Valve. J. Am. Soc. Echocardiogr. 2007, 20, 1316.e5–1316.e7. [Google Scholar] [CrossRef]
- Yutani, C.; Imakita, M.; Ishibashi-Ueda, H.; Nakamura, Y.; Sekino, T.; Iida, K.; Hisaki, R. Pulmonary Thromboembolic Hypertension in Systemic Lupus Erythematosus with Lupus Anticoagulant: Histopathological Analysis of Localization and Distribution of Thromboemboli in Pulmonary Vasculature. Intern. Med. 1995, 34, 1030–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laufer, J.; Frand, M.; Milo, S. Valve replacement for severe tricuspid regurgitation caused by Libman-Sacks endocarditis. Br. Heart J. 1982, 48, 294–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unic, D.; Planinc, M.; Baric, D.; Rudez, I.; Blazekovic, R.; Senjug, P.; Sutlic, Z. Isolated Tricuspid Valve Libman-Sacks Endocarditis in Systemic Lupus Erythematosus with Secondary Antiphospholipid Syndrome. Tex. Heart Inst. J. 2017, 44, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Hou, J.; Ren, W.; Guo, Y. Diagnosis and surgical treatment for isolated tricuspid libman-sacks endocarditis: A rare case report and literatures review. J. Cardiothorac. Surg. 2015, 10, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ma, C.; Yang, J.; Liu, S.; Zhang, Y.; Zhao, L.; Guan, Z.; Wei, H.; Gu, T. Libman-sacks endocarditis exclusively involving the tricuspid valve in a patient with systemic lupus erythematosus. J. Clin. Ultrasound 2015, 43, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Gur, A.K.; Odabasi, D.; Kunt, A.G.; Kunt, A.S. Isolated Tricuspid Valve Repair for Libman-Sacks Endocarditis. Echocardiography 2014, 31, E166–E168. [Google Scholar] [CrossRef]
- Ledingham, S.J.; Pellegrini, A.; Deverall, P.B. Bioprosthetic valve excision without replacement in the tricuspid position in a patient with Libman-Sacks endocarditis. J. Cardiovasc. Surg. 1988, 29, 356–359. [Google Scholar]
- Arif, R.; Farag, M.; Seppelt, P.; Beller, C.J.; Ruhparwar, A.; Karck, M.; Kallenbach, K. Patients with systemic lupus erythematosus and antiphospholipid syndrome undergoing cardiac valve surgery. J. Heart Valve Dis. 2015, 24, 228–235. [Google Scholar]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef]
- Özkan, M.; Çakal, B.; Karakoyun, S.; Gürsoy, O.M.; Çevik, C.; Kalçık, M.; Oğuz, A.E.; Gündüz, S.; Astarcioglu, M.A.; Aykan, A.; et al. Thrombolytic Therapy for the Treatment of Prosthetic Heart Valve Thrombosis in Pregnancy with Low-Dose, Slow Infusion of Tissue-Type Plasminogen Activator. Circulation 2013, 128, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Arnoni, R.T.; Arnoni, A.S.; Bonini, R.C.; de Almeida, A.F.; A Neto, C.; Dinkhuysen, J.J.; Issa, M.; Chaccur, P.; Paulista, P.P. Risk factors associated with cardiac surgery during pregnancy. Ann. Thorac. Surg. 2003, 76, 1605–1608. [Google Scholar] [CrossRef]
- Shook, L.L.; Barth, W.H. Cardiac Surgery during Pregnancy. Clin. Obstet. Gynecol. 2020, 63, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Sepehripour, A.H.; Lo, T.T.; Shipolini, A.R.; McCormack, D.J. Can pregnant women be safely placed on cardiopulmonary bypass? Interact. Cardiovasc. Thorac. Surg. 2012, 15, 1063–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deppisch, L.M.; Fayemi, A.O. Non-bacterial thrombotic endocarditis: Clinicopathologic correlations. Am. Heart J. 1976, 92, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Erton, Z.B.; Sevim, E.; de Jesús, G.R.; Cervera, R.; Ji, L.; Pengo, V.; Ugarte, A.; Andrade, D.; Andreoli, L.; Atsumi, T.; et al. Pregnancy outcomes in antiphospholipid antibody positive patients: Prospective results from the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (‘Registry’). Lupus Sci. Med. 2022, 9, e000633. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorini, S.; Santoro, C.; Scatteia, A.; Dellegrottaglie, S.; Tufano, A.; Cuomo, V.; Pilato, E.; Comentale, G.; D’Armiento, M.; Guida, M.; et al. A Rare Case of Tricuspid Valve Libman–Sacks Endocarditis in a Pregnant Woman with Primary Antiphospholipid Syndrome. J. Clin. Med. 2022, 11, 5875. https://doi.org/10.3390/jcm11195875
Migliorini S, Santoro C, Scatteia A, Dellegrottaglie S, Tufano A, Cuomo V, Pilato E, Comentale G, D’Armiento M, Guida M, et al. A Rare Case of Tricuspid Valve Libman–Sacks Endocarditis in a Pregnant Woman with Primary Antiphospholipid Syndrome. Journal of Clinical Medicine. 2022; 11(19):5875. https://doi.org/10.3390/jcm11195875
Chicago/Turabian StyleMigliorini, Sonia, Ciro Santoro, Alessandra Scatteia, Santo Dellegrottaglie, Antonella Tufano, Vittoria Cuomo, Emanuele Pilato, Giuseppe Comentale, Maria D’Armiento, Maurizio Guida, and et al. 2022. "A Rare Case of Tricuspid Valve Libman–Sacks Endocarditis in a Pregnant Woman with Primary Antiphospholipid Syndrome" Journal of Clinical Medicine 11, no. 19: 5875. https://doi.org/10.3390/jcm11195875




