The Present and Future of Clinical Management in Metastatic Breast Cancer
Abstract
1. Introduction
2. Molecular Classification of Breast Cancer
3. Epidemiology and Predictive/Prognostic Factors of mBC
4. Current Treatment Options of mBC
4.1. Treatment of Hormone Receptor Positive mBC
4.2. Treatment of HER-2 Positive mBC
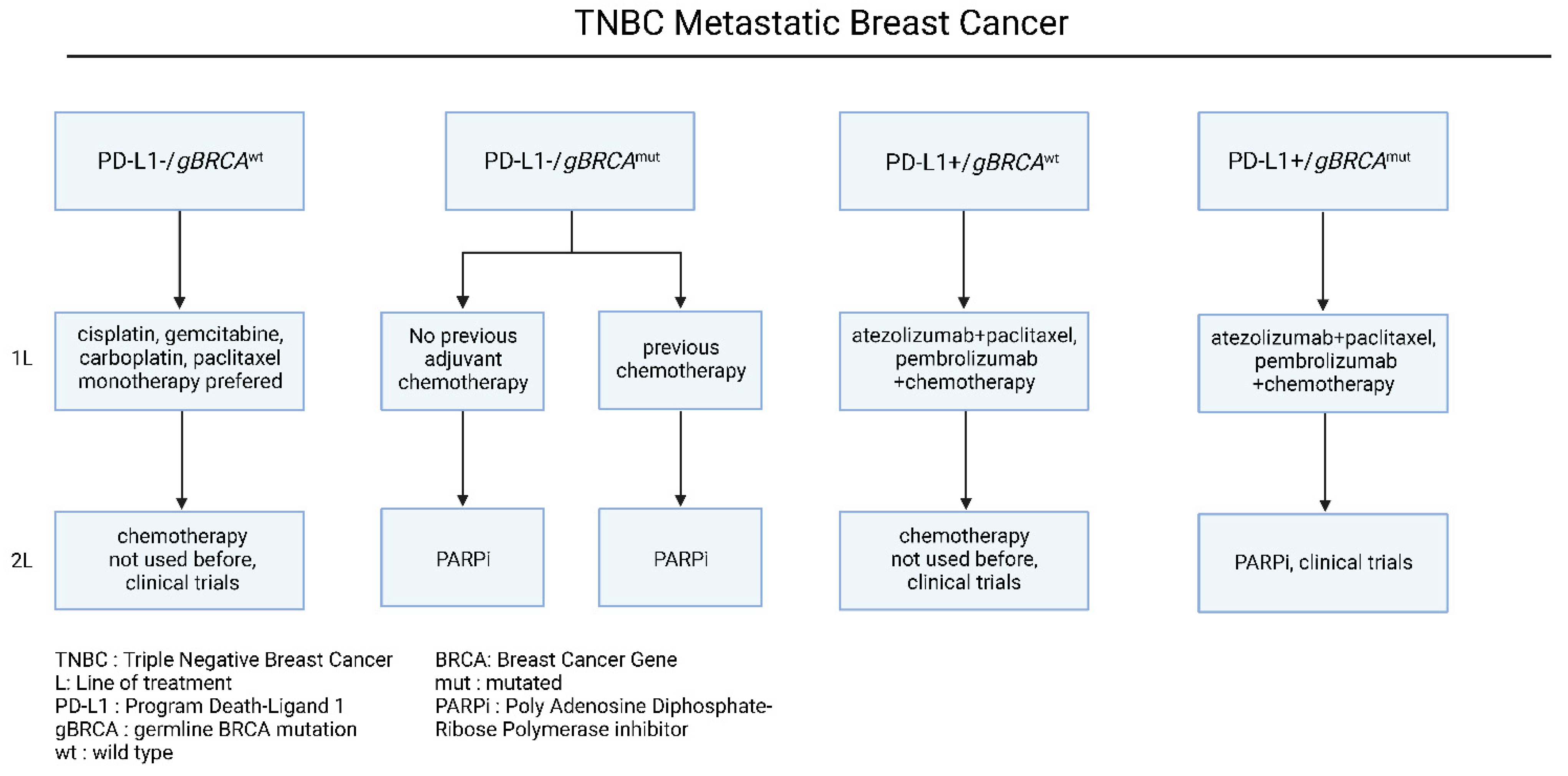
4.3. Treatment of Triple Negative mBC
5. Emerging Therapies and Clinical Trials for mBC
6. Conclusions/Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Oluwasanu, M.; Olopade, O.I. Global disparities in breast cancer outcomes: New perspectives, widening inequities, unanswered questions. Lancet Glob. Health 2020, 8, e978–e979. [Google Scholar] [CrossRef]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Öztürk, V.S.; Polat, Y.D.; Soyder, A.; Tanyeri, A.; Karaman, C.Z.; Taşkın, F. The Relationship Between MRI Findings and Molecular Subtypes in Women With Breast Cancer. Curr. Probl. Diagn. Radiol. 2020, 49, 417–421. [Google Scholar] [CrossRef]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Li, A.; Schleicher, S.M.; Andre, F.; Mitri, Z.I. Genomic alteration in metastatic breast cancer and its treatment. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 30–43. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Schiemann, W.P. Introduction to this special issue “Breast Cancer Metastasis”. J. Cancer Metastasis Treat. 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.F.R.; Fulton, R.; McLellan, M.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.; Fulton, L.; Dooling, D.; Ding, L.; Mardis, E.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Petri, B.J.; Klinge, C.M. Regulation of breast cancer metastasis signaling by miRNAs. Cancer Metastasis Rev. 2020, 39, 837–886. [Google Scholar] [CrossRef]
- Mariotto, A.B.; Etzioni, R.; Hurlbert, M.; Penberthy, L.; Mayer, M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol. Prev. Biomark. 2017, 26, 809–815. [Google Scholar] [CrossRef]
- Daily, K.; Douglas, E.; Romitti, P.A.; Thomas, A. Epidemiology of De Novo Metastatic Breast Cancer. Clin. Breast Cancer 2021. [Google Scholar] [CrossRef]
- Anderson, B.O.; Cazap, E.; El Saghir, N.S.; Yip, C.H.; Khaled, H.M.; Otero, I.V.; Adebamowo, C.A.; Badwe, R.A.; Harford, J.B. Optimisation of breast cancer management in low-resource and middle-resource countries: Executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011, 12, 387–398. [Google Scholar] [CrossRef]
- Francies, F.Z.; Hull, R.; Khanyile, R.; Dlamini, Z. Breast cancer in low-middle income countries: Abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020, 10, 1568–1591. [Google Scholar]
- Medeiros, B.; Allan, A.L. Molecular Mechanisms of Breast Cancer Metastasis to the Lung: Clinical and Experimental Perspectives. Int. J. Mol. Sci. 2019, 20, 2272. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.A.; Hortobagyi, G.N.; Smith, T.L.; Ziegler, L.D.; Frye, D.K.; Buzdar, A.U. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1996, 14, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Oncol. 2018, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.; Burstein, H.; Vora, R.S. Prognostic and Predictive Factors in Metastatic Breast Cancer. UpToDate. Burden 20. 2020. Available online: https://www.uptodate.com/contents/prognostic-and-predictive-factors-in-metastatic-breast-cancer#:~:text=by%20definition%2C%20a%20prognostic%20factor (accessed on 15 August 2022).
- Gasparini, G.; Pozza, F.; Harris, A.L. Evaluating the potential usefulness of new prognostic and predictive indicators on node-negative breast cancer patients. JNCI J. Natl. Cancer Inst. 1993, 85, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F.; Trock, B.; Harris, A.L. Assessing the clinical impact of prognostic factors: When is “statistically significant” clinically useful? Breast Cancer Res. Treat. 1998, 52, 305–319. [Google Scholar] [CrossRef]
- Swenerton, K.D.; Legha, S.S.; Smith, T.; Hortobagyi, G.N.; Gehan, E.A.; Yap, H.Y.; Gutterman, J.U.; Blumenschein, G.R. Prognostic factors in metastatic breast cancer treated with combination chemotherapy. Cancer Res. 1979, 39, 1552–1562. [Google Scholar]
- Hortobagyi, G.N.; Smith, T.L.; Legha, S.S.; Swenerton, K.D.; Gehan, E.A.; Yap, H.Y.; Buzdar, A.U.; Blumenschein, G.R. Multivariate analysis of prognostic factors in metastatic breast cancer. J. Clin. Oncol. 1983, 1, 776–786. [Google Scholar] [CrossRef]
- Clark, G.M.; Sledge, G.W., Jr.; Osborne, C.K.; McGuire, W.L. Survival from first recurrence: Relative importance of prognostic factors in 1,015 breast cancer patients. J. Clin. Oncol. 1987, 5, 55–61. [Google Scholar] [CrossRef]
- Harris, J.R.; Hellman, S. Observations on survival curve analysis with particular reference to breast cancer treatment. Cancer 1986, 57, 925–928. [Google Scholar] [CrossRef]
- Robertson, J.F.; Dixon, A.R.; Nicholson, R.I.; Ellis, I.O.; Elston, C.W.; Blamey, R.W. Confirmation of a prognostic index for patients with metastatic breast cancer treated by endocrine therapy. Breast Cancer Res. Treat. 1992, 22, 221–227. [Google Scholar] [CrossRef]
- Barrios, C.H.; Sampaio, C.; Vinholes, J.; Caponero, R. What is the role of chemotherapy in estrogen receptor-positive, advanced breast cancer? Ann. Oncol. 2009, 20, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Harris, R.; Shadbolt, B.; Palmqvist, C.; Chaudri Ross, H.A. The prognostic significance of single hormone receptor positive metastatic breast cancer: An analysis of three randomised phase III trials of aromatase inhibitors. Breast 2009, 18, 351–355. [Google Scholar] [CrossRef]
- Emi, Y.; Kitamura, K.; Shikada, Y.; Kakeji, Y.; Takahashi, I.; Tsutsui, S. Metastatic breast cancer with HER2/neu-positive cells tends to have a morbid prognosis. Surgery 2002, 131 (Suppl. 1), S217–S221. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Khan, R.; Bui, M.M. A review of triple-negative breast cancer. Cancer Control 2010, 17, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Watanabe, T.; Katsumata, N.; Omuro, Y.; Ando, M.; Fukuda, H.; Takue, Y.; Narabayashi, M.; Adachi, I.; Takashima, S. Construction and validation of a practical prognostic index for patients with metastatic breast cancer. J. Clin. Oncol. 1998, 16, 2401–2408. [Google Scholar] [CrossRef]
- Bidard, F.C.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O’Rourke, M.A.; Lew, D.L.; et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 2014, 32, 3483–3489. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, C.; Wan, S.; Mu, Z.; Zhang, Z.; Abu-Khalaf, M.M.; Fellin, F.M.; Silver, D.P.; Neupane, M.; Jaslow, R.J.; et al. Association of clinical outcomes in metastatic breast cancer patients with circulating tumour cell and circulating cell-free DNA. Eur. J Cancer 2019, 106, 133–143. [Google Scholar] [CrossRef]
- McAndrew, N.P.; Finn, R.S. Clinical review on the management of hormone receptor–positive metastatic breast cancer. JCO Oncol. Pract. 2022, 18, 319–327. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef]
- Taylor, C.W.; Green, S.; Dalton, W.S.; Martino, S.; Rector, D.; Ingle, J.N.; Robert, N.J.; Budd, G.T.; Paradelo, J.C.; Natale, R.B.; et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: An intergroup study. J. Clin. Oncol. 1998, 16, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Solovieff, N.; Andre, F.; O’Shaughnessy, J.; Cameron, A.; Janni, W.; Sonke, G.; Yap, Y.; Yardley, D.; Zarate, J.; et al. Correlative analysis of overall survival by intrinsic subtype across the MONALEESA-2, -3, and -7 studies of ribociclib + endocrine therapy in patients with HR+/HER2− advanced breast cancer. In Proceedings of the 2021 San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 7–10 December 2021. [Google Scholar]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Linden, H.M.; et al. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N. Engl. J. Med. 2019, 380, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.; Jönsson, P.E.; Lidbrink, E.K.; Trudeau, M.; Eiermann, W.; Brattström, D.; Lindemann, J.P.; Wiklund, F.; Henriksson, R. FACT: An open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J. Clin. Oncol. 2012, 30, 1919–1925. [Google Scholar] [CrossRef]
- Mauri, D.; Pavlidis, N.; Polyzos, N.P.; Ioannidis, J.P. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: Meta-analysis. J. Natl. Cancer Inst. 2006, 98, 1285–1291. [Google Scholar] [CrossRef]
- Kornblum, N.; Zhao, F.; Manola, J.; Klein, P.; Ramaswamy, B.; Brufsky, A.; Stella, P.J.; Burnette, B.; Telli, M.; Makower, D.F.; et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J. Clin. Oncol. 2018, 36, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Piccart-Gebhart, M.J.; Burzykowski, T.; Buyse, M.; Sledge, G.; Carmichael, J.; Lück, H.-J.; Mackey, J.R.; Nabholtz, J.-M.; Paridaens, R.; Biganzoli, L.; et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J. Clin. Oncol. 2008, 26, 1980–1986. [Google Scholar] [CrossRef]
- Chan, S.; Romieu, G.; Huober, J.; Delozier, T.; Tubiana-Hulin, M.; Schneeweiss, A.; Lluch, A.; Llombart, A.; du Bois, A.; Kreienberg, R.; et al. Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. J. Clin. Oncol. 2009, 27, 1753–1760. [Google Scholar] [CrossRef]
- Thomas, E.S. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J. Clin. Oncol. 2008, 26, 2223. [Google Scholar] [CrossRef]
- Martínez-Sáez, O.; Prat, A. Current and future management of HER2-positive metastatic breast cancer. JCO Oncol. Pract. 2021, 17, 594–604. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Gobbini, E.; Ezzalfani, M.; Dieras, V.; Bachelot, T.; Brain, E.; Debled, M.; Jacot, W.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur. J. Cancer 2018, 96, 17–24. [Google Scholar] [CrossRef]
- Baselga, J.; Swain, S.M. CLEOPATRA: A phase III evaluation of pertuzumab and trastuzumab for HER2-positive metastatic breast cancer. Clin. Breast Cancer 2010, 10, 489–491. [Google Scholar] [CrossRef]
- Scheuer, W.; Friess, T.; Burtscher, H.; Bossenmaier, B.; Endl, J.; Hasmann, M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009, 69, 9330–9336. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Yu, X.; Bai, Y.; McBride, H.J.; Huang, X. Cryo-EM Structure of HER2-trastuzumab-pertuzumab complex. PLoS ONE 2019, 14, e0216095. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Ciruelos, E.; Schneeweiss, A.; Puglisi, F.; Peretz-Yablonski, T.; Bondarenko, I.; Paluch-Shimon, S.; Wardley, A.; Merot, J.L.; Du Toit, Y.; et al. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann. Oncol. 2019, 30, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; L’opez-Vega, J.M.; Petit, T.; Zamagni, C.; Easton, V.; Kamber, J.; Restuccia, E.; Andersson, M. Safety and efficacy of vinorelbine in combination with pertuzumab and trastuzumab for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer: VELVET cohort 1 final results. Breast Cancer Res. 2016, 18, 126. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Diéras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.M.; Smitt, M.; Yu, R.; Leung, A.C.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.B.; Martin, A.G.; LoRusso, P.M.; Ferrero, J.M.; Badovinac-Crnjevic, T.; Hoersch, S.; Smitt, M.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017, 18, 743–754. [Google Scholar] [CrossRef]
- Pheneger, T.; Bouhana, K.; Anderson, D.; Garrus, J.; Ahrendt, K.; Allen, S. In vitro and in vivo activity of ARRY-380: A potent, small molecule inhibitor of ErbB2. In Proceedings of the American Association for Cancer Research, Denver, CO, USA, 18–22 April 2009. [Google Scholar]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol. 2020, 38, 2610. [Google Scholar] [CrossRef] [PubMed]
- Wardley, A.; Mueller, V.; Paplomata, E.; Crouzet, L.; Iqbal, N.; Aithal, S.; Block, M.; Cold, S.; Hahn, O.; Poosarla, T.; et al. Abstract PD13-04: Impact of tucatinib on health-related quality of life in patients with HER2 metastatic breast cancer with stable and active brain metastases. In Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium, San Antonio, TX, USA, 8–11 December 2020. [Google Scholar]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Balduzzi, S.; Mantarro, S.; Guarneri, V.; Tagliabue, L.; Pistotti, V.; Moja, L.; D’Amico, R. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst. Rev. 2014, 2014, CD006242. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Nagpal, A.; Redvers, R.P.; Ling, X.; Ayton, S.; Fuentes, M.; Tavancheh, E.; Diala, I.; Lalani, A.; Loi, S.; David, S.; et al. Neoadjuvant neratinib promotes ferroptosis and inhibits brain metastasis in a novel syngeneic model of spontaneous HER2+ve breast cancer metastasis. Breast Cancer Res. 2019, 21, 94. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥2 HER2-directed regimens: Phase III NALA trial. J. Clin. Oncol. 2020, 38, 3138. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Baselga, J. Trastuzumab-DM1: Building a chemotherapy-free road in the treatment of human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2011, 29, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Barcenas, C.H.; Hurvitz, S.A.; Di Palma, J.A.; Bose, R.; Chien, A.J.; Iannotti, N.; Marx, G.; Brufsky, A.; Litvak, A.; Ibrahim, E.; et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: The CONTROL trial. Ann. Oncol. 2020, 31, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Lambertini, M.; de Azambuja, E. How I treat metastatic triple-negative breast cancer. ESMO Open 2019, 4, e000504. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Won, K.A.; Spruck, C. Triple negative breast cancer therapy: Current and future perspectives. Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Adel, N.G. Current treatment landscape and emerging therapies for metastatic triple-negative breast cancer. Am. J. Manag. Care 2021, 27 (Suppl. 5), S87–S96. [Google Scholar]
- Zeichner, S.B.; Terawaki, H.; Gogineni, K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer Basic Clin. Res. 2016, 10, BCBCR-S32783. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Kieffer, S.R.; Lowndes, N.F. Immediate-Early, Early, and Late Responses to DNA Double Stranded Breaks. Front. Genet. 2022, 13, 793884. [Google Scholar] [CrossRef]
- Richard, I.A.; Burgess, J.T.; O’Byrne, K.J.; Bolderson, E. Beyond PARP1: The Potential of Other Members of the Poly (ADP-Ribose) Polymerase Family in DNA Repair and Cancer Therapeutics. Front. Cell Dev. Biol. 2022, 9, 801200. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Bajrami, I.; Taniguchi, T.; Lord, C.J. Synthetic lethality: The road to novel therapies for breast cancer. Endocr.-Relat. Cancer 2016, 23, T39–T55. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A.; Lord, C.J. Synthetic lethal therapies for cancer: What’s next after PARP inhibitors? Nat. Rev. Clin. Oncol. 2018, 15, 564–576. [Google Scholar] [CrossRef]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, M.; Jiang, Z.; Wang, X. A comprehensive immunologic portrait of triple-negative breast cancer. Transl. Oncol. 2018, 11, 311–329. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumor-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Rostami, R.; Mittal, S.; Rostami, P.; Tavassoli, F.; Jabbari, B. Brain metastasis in breast cancer: A comprehensive literature review. J. Neurooncol. 2016, 127, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.N.; Figura, N.B.; Arrington, J.A.; Yu, H.H.M.; Etame, A.B.; Vogelbaum, M.A.; Soliman, H.; Czerniecki, B.J.; Forsyth, P.A.; Han, H.S.; et al. Management of brain metastases in breast cancer: A review of current practices and emerging treatments. Breast Cancer Res. Treat. 2020, 180, 279–300. [Google Scholar] [CrossRef]
- Soffietti, R.; Abacioglu, U.; Baumert, B.; Combs, S.E.; Kinhult, S.; Kros, J.M.; Marosi, C.; Metellus, P.; Radbruch, A.; Villa Freixa, S.S.; et al. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European Association of Neuro-Oncology (EANO). Neuro-Oncology 2017, 19, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Burri, S.H.; Asher, A.L.; Crocker, I.R.; Fraser, R.W.; Zhang, C.; Chen, Z.; Kandula, S.; Zhong, J.; Press, R.H.; et al. Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: A multi-institutional analysis. Neurosurgery 2016, 79, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-Oncology 2013, 15, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Deshmukh, S.; Brown, P.D.; Wefel, J.S.; Tome, W.A.; Bruner, D.W.; Bovi, J.A.; Robinson, C.G.; Khuntia, D.; Grosshans, D.R.; et al. Preservation of neurocognitive function (NCF) with conformal avoidance of the hippocampus during whole-brain radiotherapy (HA-WBRT) for brain metastases: Preliminary results of phase III trial NRG oncology CC001. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1607. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomized, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Kayama, T.; Sato, S.; Sakurada, K.; Mizusawa, J.; Nishikawa, R.; Narita, Y.; Sumi, M.; Miyakita, Y.; Kumabe, T.; Sonoda, Y.; et al. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): A phase III, noninferiority, randomized controlled trial. J. Clin. Oncol. 2018, 36, 3282–3289. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Sahgal, A.; Aoyama, H.; Kocher, M.; Neupane, B.; Collette, S.; Tago, M.; Shaw, P.; Beyene, J.; Chang, E.L. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: Individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.-M.; WiIdrick, D.M. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 1998, 42, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.; Osorio, D.; Expósito Hernandez, J.; Simancas-Racines, D.; Martinez-Zapata, M.J.; Bonfill Cosp, X. Surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis. Cochrane Database Syst. Rev. 2018, 8, CD012086. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, T.; Sombeck, M.; Sollaccio, R. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. Curr. Oncol. Rep. 2011, 13, 255–258. [Google Scholar] [CrossRef]
- Chernov, M.F.; Nakaya, K.; Izawa, M.; Hayashi, M.; Usuba, Y.; Kato, K.; Muragaki, Y.; Iseki, H.; Hori, T.; Takakura, K. Outcome after radiosurgery for brain metastases in patients with low Karnofsky performance scale (KPS) scores. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1492–1498. [Google Scholar] [CrossRef]
- Robertson, J.F.; Evans, A.; Henschen, S.; Kirwan, C.C.; Jahan, A.; Kenny, L.M.; Dixon, J.M.; Schmid, P.; Kothari, A.; Mohamed, O.; et al. A randomized, open-label, presurgical, window-of-opportunity study comparing the pharmacodynamic effects of the novel oral SERD AZD9496 with fulvestrant in patients with newly diagnosed ER+ HER2− primary breast cancer. Clin. Cancer Res. 2020, 26, 4242–4249. [Google Scholar] [CrossRef]
- Wander, S.A.; Han, H.S.; Zangardi, M.L.; Niemierko, A.; Mariotti, V.; Kim, L.S.; Xi, J.; Pandey, A.; Dunne, S.; Nasrazadani, A.; et al. Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: A multicenter experience. J. Natl. Compr. Cancer Netw. 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Rugo, H.S.; Delord, J.P.; Im, S.A.; Ott, P.A.; Piha-Paul, S.A.; Bedard, P.L.; Sachdev, J.; Tourneau, C.L.; van Brummelen, E.M.; Varga, A.; et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. Clin. Cancer Res. 2018, 24, 2804–2811. [Google Scholar] [CrossRef]
- Esteva, F.J.; Hubbard-Lucey, V.M.; Tang, J.; Pusztai, L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019, 20, e175–e186. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Dickler, M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist 2018, 23, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Soleja, M.; Raj, G.V.; Unni, N. An evaluation of fulvestrant for the treatment of metastatic breast cancer. Expert Opin. Pharmacother. 2019, 20, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.P.; Patel, M.R.; Armstrong, A.C.; Baird, R.D.; Jhaveri, K.; Hoch, M.; Klinowska, T.; Lindemann, J.P.O.; Morgan, S.R.; Schiavon, G.; et al. A First-in-Human Study of the New Oral Selective Estrogen Receptor Degrader AZD9496 for ER+/HER2− Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Mavratzas, A.; Marmé, F. Treatment of Luminal Metastatic Breast Cancer beyond CDK4/6 Inhibition: Is There a Standard of Care in Clinical Practice? Breast Care (Basel) 2021, 16, 115–128. [Google Scholar] [CrossRef]
- Uzhachenko, R.V.; Bharti, V.; Ouyang, Z.; Blevins, A.; Mont, S.; Saleh, N.; Lawrence, H.A.; Shen, C.; Chen, S.C.; Ayers, G.D.; et al. Metabolic modulation by CDK4/6 inhibitor promotes chemokine-mediated recruitment of T cells into mammary tumors. Cell Rep. 2021, 35, 108944, Erratum in Cell Rep. 2021, 35, 109271. [Google Scholar] [CrossRef]
- Hyder, T.; Marti, J.L.G.; Nasrazadani, A.; Brufsky, A.M. Statins and endocrine resistance in breast cancer. Cancer Drug Resist. 2021, 4, 356–364. [Google Scholar] [CrossRef]
- Disis, M.L.; Stanton, S.E. Triple-negative breast cancer: Immune modulation as the new treatment paradigm. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e25–e30. [Google Scholar] [CrossRef]
- Yang, L.; Ning, Q.; Tang, S.S. Recent Advances and Next Breakthrough in Immunotherapy for Cancer Treatment. J. Immunol. Res. 2022, 2022, 8052212. [Google Scholar] [CrossRef]
- Guda, P.; Chittur, S.V.; Guda, C. Comparative analysis of protein-protein interactions in cancer-associated genes. Genom. Proteom. Bioinform. 2009, 7, 25–36. [Google Scholar] [CrossRef]
- Barchiesi, G.; Roberto, M.; Verrico, M.; Vici, P.; Tomao, S.; Tomao, F. Emerging Role of PARP Inhibitors in Metastatic Triple Negative Breast Cancer. Current Scenario and Future Perspectives. Front. Oncol. 2021, 11, 769280. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Antrás, J.; Cescon, D.W. PARP inhibitor sensitivity in BRCA-related metastatic breast cancer: An OlympiAD later. Ann. Oncol. 2021, 32, 1460–1462. [Google Scholar] [CrossRef] [PubMed]





| Prognostic Factors | Details | References |
|---|---|---|
| Relapse Free interval | ≥2 years from primary breast cancer diagnosis is considered more favorable | Swenerton et al. [27] Hortobagyi et al. [28] Clark et al. [29] Harris et al. [30] |
| Metastatic sites: bones, chest wall, or lymph nodes | May have prolonged-free survival | Swenerton et al. [27] Hortobagyi et al. [28] Robertson et al. [31] |
| Metastatic sites: hepatic or lymphangitic pulmonary disease | Shorter PFS and OS | Barrios et al. [32] |
| Hormone receptor status | HR+: more favorable prognosis, ER+/PR+: significantly longer survival than single hormone receptor-positive tumors | Stuart-Harris et al. [33] |
| HER-2+ or TNBC | Shorter median survival | Clark et al. [29] Emi et al. [34] Ismail-Khan et al. [35] |
| PS (Performance Status) | Weight loss, high LDH and low PS are poor prognostic features | Swenerton et al. [27] Yamamoto et al. [36] |
| Circulating Tumor Cells (CTC) * | CTC ≥ 5/7.5 mL, poor prognosis with shortened PFS and OS | Bidard et al. [37] Smerage et al. [38] |
| Circulating tumor DNA (ctDNA) * | High ctDNA, increased risk of death | Ye et al. [39] |
| Indication | Therapy |
|---|---|
| Single, surgically accessible metastasis with favorable prognosis | Surgical resection [95,96,97,98,99,100] Whole brain radiotherapy (WBRT) [101] |
| Single, surgically inaccessible metastasis with favorable prognosis | Stereotactic Radiosurgery (SRS) with WBRT [102,103,104,105] |
| Multiple < 3 cm brain metastases, with favorable prognosis | SRS alone [106] Adjunctive WBRT [107] |
| Poor prognosis/PS | WBRT vs. SRS [108,109] |
| Patients with progressive extracranial disease or no feasible local therapy option | Systemic therapy based on subtypes [110] |
| Subtype | Drug/Trial Name | Drug Target | Phase | HR (PFS/OS) | Trial Number/Status |
|---|---|---|---|---|---|
| HR+ | |||||
| Fulvestrant + AZD9496 | SERD | I | - | NCT03236974 (completed) [111] | |
| Elacestrant (EMERALD) | SERD | III | - | NCT03778931 (ongoing) | |
| Giredestrant (GDC-9545) + Palbociclib | SERD | I | - | NCT03332797 (ongoing) | |
| Amcenestrant + fulvestrant | SERD | II | - | NCT04059484 (ongoing) | |
| Camizestrant (AZD9833) | SERD | II | - | NCT04214288 (ongoing) | |
| G1T48 + Palbociclib | SERD | I | - | NCT03455270 (ongoing) | |
| AC682 | SERD | I | - | NCT05080842 (ongoing) | |
| H3B-6545 | SERCA | I/II | - | NCT03250676 (ongoing) | |
| Atorvastatin (MASTER) | HMG-CoA reductase | III | - | NCT04601116 (ongoing) | |
| Onapristone + fulvestrant (SMILE) | Type I antiprogestin | II | - | NCT04738292 (ongoing) | |
| Hemay022 + endocrine therapy | Irreversible EGFR inhibitor | I | - | NCT03308201 (ongoing) | |
| ARV-471 | PROTAC | I/II | - | NCT04072952 (ongoing) | |
| AZD5363 + fulvestrant | AKTi | I/II | - | NCT01992952 (ongoing) | |
| Ipatasertib (GDC-0068) + fulvestrant | AKTi | III | - | NCT04650581 (ongoing) | |
| HS-10352 | PIK3-p110α | I | - | NCT04631835 (ongoing) | |
| Everolimus + Exemestane | mTORC1/2 inhibitor | II | - | NCT03312738 (ongoing) | |
| AZD2014 + Palbociclib | mTORC1/2 inhibitor | I | - | NCT02599714 (ongoing) | |
| Crizotinib + Fulvestrant | ALK/MET inhibitor | II | - | NCT03620643 (ongoing) | |
| Cabozantinib + Fulvestrant | VEGFR2, MET, RET inhibitor | II | - | NCT01441947 (ongoing) | |
| Bevacizumab + Ixabepilone | VEGF inhibitor | III | - | NCT00785291 (ongoing) | |
| Zilovertamab vedotin (MK-2140) | ROR1 inhibitor | II | - | NCT04504916 (ongoing) | |
| Infigratinib + Palbociclib + Fulvestrant | FGFRi + CDK4/6i | Ib | - | NCT04504331 (ongoing) | |
| Ε7090 + Fulvestrant | FGFRi | Ι | - | NCT04572295 (ongoing) | |
| Bortezomib + fulvestrant | Proteasome inhibitor | II | - | NCT01142401 (ongoing) | |
| trifluridine/tipiracil (TAS-102) (TIBET) | nucleoside analog plus thymidine phosphorylase inhibitor | II | - | NCT04489173 (ongoing) | |
| trastuzumab deruxtecan ** (Breast04) | ADC | III | - | NCT03734029 (ongoing) | |
| sacituzumab govitecan (TROPiCS-02) | ADC/Topo I | III | - | NCT03734029 (ongoing) | |
| Dato-DXd (TROPION-Breast01) | TROP2-directed ADC | III | - | NCT05104866 (ongoing) | |
| APG-2575 ± Palbociclib | Bcl-2 inhibitor | Ib/II | - | NCT04946864 (ongoing) | |
| ALRN-6924 + Paclitaxel | MDM2 inhibitor | I | - | NCT03725436 (ongoing) | |
| abemaciclib # | CDK4/6i | retro multicenter [112] | PFS: 5.1 vs. 5.7 m, OS: 17.2 vs. 15.3 m | - | |
| Dalpiciclib (SHR6390) | CDK4/6i | I | - | NCT04236310 (ongoing) | |
| HRS8807 + SHR6390 | CDK4/6i | I | - | NCT04993430 (ongoing) | |
| PRT2527 | CDK9 | I | - | NCT05159518 (ongoing) | |
| Pembrolizumab (KEYNOTE 028) | IO | Ib | ORR: 12% | NCT02054806 (completed) [113] | |
| Nivolumab + ipilimumab + Nab-paclitaxel | anti-PDL1 + anti-CTLA-4 | I | - | NCT04132817 (ongoing) | |
| Avelumab + Palbociclib + Endocrine therapy | IO + CDK4/6i | II | - | NCT03573648 (ongoing) | |
| Durvalumab + Olaparib + fulvestrant | anti-PDL1 + PARPi | II | - | NCT04053322 (ongoing) | |
| Tucidinostat + Exemestane | HDAC inhibitor | II | - | NCT04465097 (ongoing) | |
| Vorinostat + Pembrolizumab | HDAC inhibitor + IO | II | - | NCT04190056 (ongoing) | |
| ESR1 peptide vaccine + GM-CSF | Vaccine | I | - | NCT04270149 (ongoing) | |
| HER-2+ | |||||
| Tucatinib (HER2CLIMB) | anti-HER-2 | III | HR 0.58/0.85 | NCT02614794 (completed) | |
| MCLA-128 + trastuzumab | NRG1 fusion inhibitor | II | - | NCT03321981 (ongoing) | |
| Palbociclib + anti-HER-2 (PATINA) | CDK4/6i | III | - | NCT02947685 (ongoing) | |
| Alpelisib + anti-HER-2 (EPIK-B2) | PIK3α inhibitor | III | - | NCT04208178 (ongoing) | |
| GDC-0084 + trastuzumab | PIK3 inhibitor | II | - | NCT03765983 (ongoing) | |
| Copanlisib + trastuzumab | PIK3α inhibitor | I/II | - | NCT02705859 (ongoing) | |
| Gedatolisib + Herceptin | PIK3 inhibitor | II | - | NCT03698383 (ongoing) | |
| Ibrutinib + trastuzumab | BTK inhibitor | I/II | - | NCT03379428 (ongoing) | |
| Ceralasertib (DASH) | ATR inhibitor | I/II | - | NCT04704661 (ongoing) | |
| AUY922 + trastuzumab | HSP90 inhibitor | I/II | - | NCT01271920 (completed) | |
| Ganitumab (I-SPY) | IGF-1R inhibitor | I/II | - | NCT01042379 (ongoing) | |
| TVB-2640 + trastuzumab | FASN inhibitor | II | - | NCT03179904 (ongoing) | |
| ladiratuzumab vedotin + trastuzumab | zinc transporter LIV-1 inhibitor | I | - | NCT01969643 (ongoing) | |
| DC1 (Dendritic Cell)-WOKVAC | Vaccine | II | - | NCT03384914 (ongoing) | |
| TPIV100 | anti-HER- 2 Vaccine | II | - | NCT04197687 (ongoing) | |
| pNGVL3-hICD | anti-HER- 2 Vaccine | I | - | NCT00436254 (ongoing) | |
| KN035 + trastuzumab | Single Domain a-PD-L1 | I/II | - | NCT04034823 (ongoing) | |
| M7824 | PD-L1/TGFβ fusion protein | II | - | NCT03620201 (ongoing) | |
| PRS-343 + atezolizumab | 4-1BB Ab | Ib | - | NCT03650348 (ongoing) | |
| SBT6050 + anti-HER-2 | TLR8 agonist | I/II | - | NCT05091528 (ongoing) | |
| BPX-603 | CAR-T cells | I/II | - | NCT04650451 (ongoing) | |
| TNBC | |||||
| Goserelin | GnRH analog | II | - | NCT03444025 (ongoing) | |
| Nadunolimab + chemo | IL1RAP | I/II | - | NCT05181462 (ongoing) | |
| SKB264 | TROP2-directed ADC | III | - | NCT05347134 (ongoing) | |
| ASTX660 + pembrolizumab (ASTEROID) | IAPi + IO | I | - | NCT05082259 (ongoing) | |
| OMO-103 | anti-Myc CPP | I/II | - | NCT04808362 (ongoing) | |
| SKL27969 | PRMT5 | I/II | - | NCT05388435 (ongoing) | |
| LY3023414 + Prexasertib | PIK3/AKT + CHEK1i | II | - | NCT04032080 (ongoing) | |
| Sitravatinib | Multi-kinase inhibitor | II | - | NCT04123704 (ongoing) | |
| Tak-228 + Tak-117 + Chemo | PIK3/AKT/mTORC1i | II | - | NCT03193853 (ongoing) | |
| Eganelisib + pembrolizumab + bevacizumab + paclitaxel | PIK3/AKT/mTORC1i + IO + anti-VEGF | I/II | - | NCT05390710 (ongoing) | |
| Capivasertib + Paclitaxel (CAPItello-290) | pan-AKTi + Chemo | III | - | NCT03997123 (ongoing) | |
| Gedatolisib + Talazoparib | PIK3i + PAPRi | I/II | - | NCT03911973 (ongoing) | |
| AZD6738 + Olaparib + Durvalumab (PHOENIX) | ATRi + PARPi + IO | II | - | NCT03740893 (ongoing) | |
| Olinvacimab + pembrolizumab | anti-VEGFR2 + IO | II | - | NCT04986852 (ongoing) | |
| PMD-026 | RSKi | I | - | NCT04115306 (ongoing) | |
| Talazoparib + Selinexor (START) | PARPi + XPO1i | - | NCT05035745 (ongoing) | ||
| Chiauranib + capecitabine | Multi-kinase inhibitor | II | NCT05336721 (ongoing) | ||
| TT-00420 | Multi-kinase inhibitor | I | NCT03654547 (ongoing) | ||
| AL101 | γ-secretase NOTCHi | II | - | NCT04461600 (ongoing) | |
| ZEN003694 + Talazoparib | BET domain inhibitor + PARPi | II | - | NCT03901469 (ongoing) | |
| Binimetinib + Palbociclib | MEK1/2i + CDK4/6i | I/II | - | NCT04494958 (ongoing) | |
| Trilaciclib + Sacituzumab Govitecan | CDK4/6i + TROP-2 directed ADC | II | - | NCT05113966 (ongoing) | |
| Chidamide + chemo | HDAC | II/III | - | NCT04582955 (ongoing) | |
| Eryaspase + chemotherapy (TRYbeCA-2) | L-asparaginase | II/III | - | NCT03674242 (ongoing) | |
| Deferoxamine + chemo | Iron Binding agent | II | - | NCT05300958 (ongoing) | |
| SG001 + paclitaxel | IO | II | - | NCT05068141 (ongoing) | |
| Serplulimab + chemo | IO | III | - | NCT04301739 (ongoing) | |
| KN046 + paclitaxel | anti-PD-L1/CTLA-4 | I/II | - | NCT03872791 (ongoing) | |
| CDX-1140 + CDX-301 + PLD Chemotherapy | CD40 agonist + anti-FLT3 | I | - | NCT05029999 (ongoing) | |
| Romidepsin + nivolumab + cisplatin | HDAC + IO | I/II | - | NCT02393794 (ongoing) | |
| Tiragolumab + Atezolizumab + paclitaxel | anti-TIGIT + IO | I | - | NCT04584112 (ongoing) | |
| Fruquintinib + | anti-VEGF + IO | I/II | - | NCT04577963 (ongoing) | |
| Anlotinib + Tislelizumab | anti-VEGF/MEK + IO | II | - | NCT04914390 (ongoing) | |
| Niraparib + Dostarlimab + RT | PARPi + IO + RT | II | - | NCT04837209 (ongoing) | |
| Ipatasertib + Atezolizumab | AKTi + IO | III | - | NCT04177108 (ongoing) | |
| Magrolimab + Paclitaxel + Sacituzumab Govitecan | anti-CD47 + ADC | II | - | NCT04958785 (ongoing) | |
| CMP-001 + RT | TLR9 pDC agonist | II | - | NCT04807192 (ongoing) | |
| TIL LN-145 | Tumor Infiltrating Lymphocytes | II | - | NCT04111510 (ongoing) | |
| BDB001 + atezolizumab + RT (AGADIR) | TRL7 agonist + IO | II | - | NCT03915678 (ongoing) | |
| Spartalizumab LAG525 + NIR178 + capmatinib | A2AR antagonist + METi+ IO | I | - | NCT03742349 (ongoing) | |
| Sitravatinib + Tislelizumab | Multi-kinase inhibitor + IO | II | - | NCT04734262 (ongoing) | |
| Ivermectin + pembrolizumab | IMPα/β1 stabilizer + IO | II | - | NCT05318469 (ongoing) | |
| Tavokinogene telseplasmid + pembrolizumab | IL-12 injecting tele- monitored plasmid + IO | NCT03567720 (ongoing) | |||
| CF33-hNIS- | Oncolytic Virus- | I | - | NCT05081492 | |
| antiPDL1 | conjugated with IO | (ongoing) | |||
| RBX7455 | Microbiota-based formulation | I | - | NCT04139993 (ongoing) | |
| ADV/HSV-tk + RT + Pembrolizumab | Oncolytic Virus + RT + IO | II | - | NCT03004183 (ongoing) | |
| mRNA-275 + Durvalumab | mRNA + IO | I | - | NCT03739931 (ongoing) | |
| PVX-410 + pembrolizumab + chemo | Vaccine + IO | II | - | NCT04634747 (ongoing) | |
| AE37 + pembrolizumab | Vaccine + IO | II | - | NCT04024800 (ongoing) | |
| X4P-001 + Toripalimab | CXCR4 antagonist + IO | I/II | - | NCT05103917 (ongoing) | |
| EGFR/B7H3 CAR-T | CAR-T cells | I | - | NCT05341492 (ongoing) | |
| All subtypes | IO-based combinations | ADC, HDAC, anti-VEGF, CDK4/6i, PARP | I-III | - | Extensively reviewed [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.H.; Laliotis, G. The Present and Future of Clinical Management in Metastatic Breast Cancer. J. Clin. Med. 2022, 11, 5891. https://doi.org/10.3390/jcm11195891
Lin PH, Laliotis G. The Present and Future of Clinical Management in Metastatic Breast Cancer. Journal of Clinical Medicine. 2022; 11(19):5891. https://doi.org/10.3390/jcm11195891
Chicago/Turabian StyleLin, Pauline H., and George Laliotis. 2022. "The Present and Future of Clinical Management in Metastatic Breast Cancer" Journal of Clinical Medicine 11, no. 19: 5891. https://doi.org/10.3390/jcm11195891
APA StyleLin, P. H., & Laliotis, G. (2022). The Present and Future of Clinical Management in Metastatic Breast Cancer. Journal of Clinical Medicine, 11(19), 5891. https://doi.org/10.3390/jcm11195891





