Pulmonary Capillary Wedge Pressure during Exercise Is Prognostic for Long-Term Survival in Patients with Symptomatic Heart Failure †
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Right Heart Catheterization and Exercise Testing
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
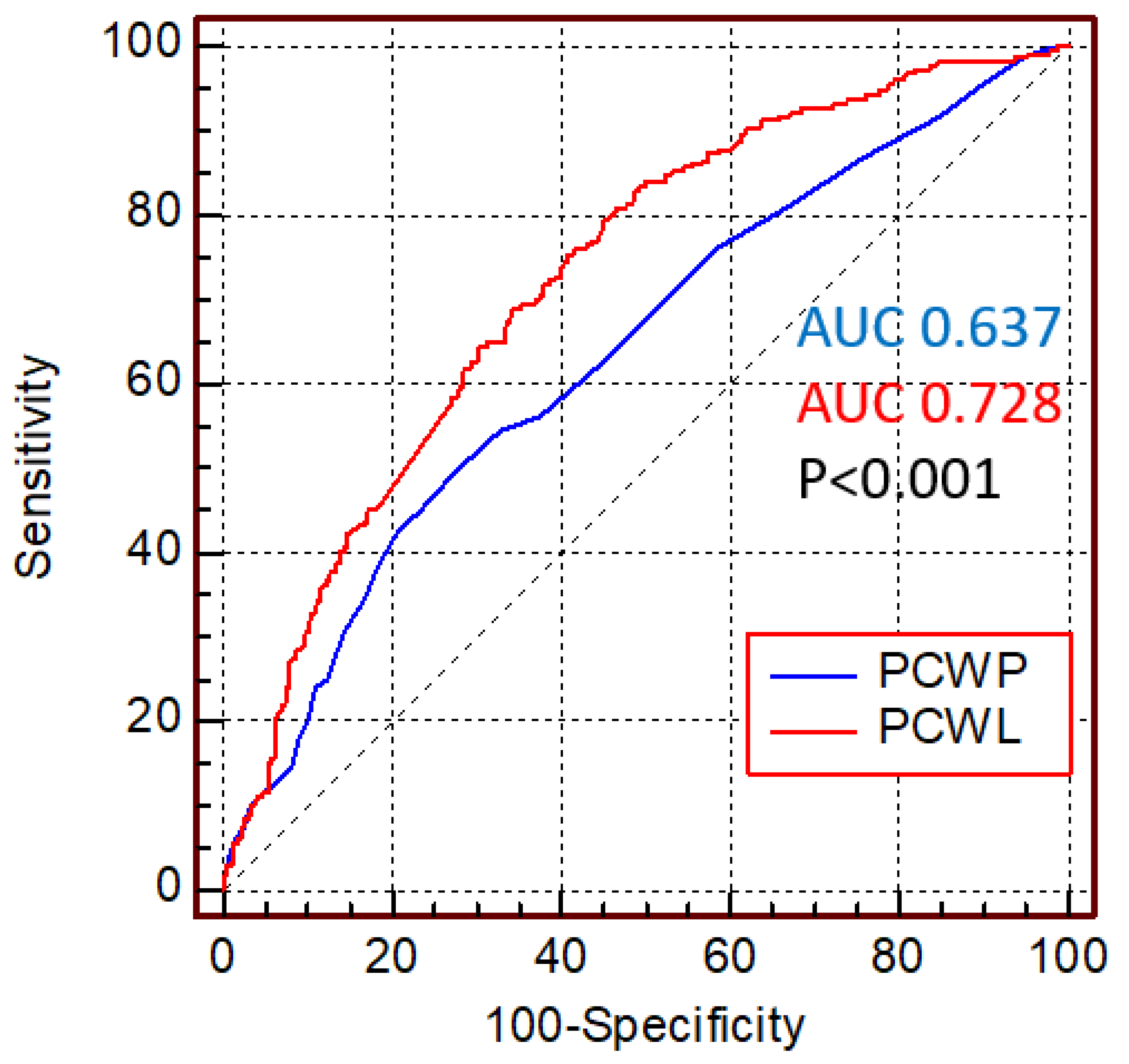
3.2. Pulmonary Capillary Wedge Pressure and Survival
3.3. Pulmonary Capillary Wedge Pressure during Exercise and Survival in Heart Failure Stratified by Left Ventricular Systolic Function
4. Discussion
4.1. Hemodynamics of Heart Failure
4.2. Left Ventricular Function and Death in Heart Failure
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Eur. J. Heart Fail. 2021, 27, 387–413. [Google Scholar]
- Henkel, D.M.; Redfield, M.M.; Weston, S.A.; Gerber, Y.; Roger, V.L. Death in heart failure: A community perspective. Circ. Heart Fail. 2008, 1, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.-P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018, 6, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Dorfs, S.; Zeh, W.; Hochholzer, W.; Jander, N.; Kienzle, R.P.; Pieske, B.; Neumann, F.J. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur. Heart J. 2014, 44, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Roul, G.; Moulichon, M.E.; Bareiss, P.; Gries, P.; Koegler, A.; Sacrez, J.; Germain, P.; Mossard, J.M.; Sacrez, A. Prognostic factors of chronic heart failure in NYHA class II or III: Value of invasive exercise haemodynamic data. Eur. Heart J. 1995, 16, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Thompson, B.R.; Brunner-La Rocca, H.P.; Kaye, D.M. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J. Am. Coll. Cardiol. 2010, 56, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.J.; d’Agostino, R.B.; d’Agostino, R.B.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transpl. 2016, 35, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Eisman, A.S.; Shah, R.V.; Dhakal, B.P.; Pappagianopoulos, P.P.; Wooster, L.; Bailey, C.; Cunningham, T.F.; Hardin, K.M.; Baggish, A.L.; Ho, J.E.; et al. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ. Heart Fail. 2018, 11, e004750. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.P.; Johnson, B.D.; Borlaug, B.A. Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.P.; Malhotra, R.; Murphy, R.M.; Pappagianopoulos, P.P.; Baggish, A.L.; Weiner, R.B.; Houstis, N.E.; Eisman, A.S.; Hough, S.S.; Lewis, G.D. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circ. Heart Fail. 2015, 8, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Stolfo, D.; Sinagra, G.; Lund, L.H. Heart failure with mid-range or mildly reduced ejection fraction. Nat. Rev. Cardiol. 2022, 19, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.P.; Sokol, S.I.; Wang, Y.; Rathore, S.S.; Ko, D.T.; Jadbabaie, F.; Portnay, E.L.; Marshalko, S.J.; Radford, M.J.; Krumholz, H.M. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J. Am. Coll. Cardiol. 2003, 42, 736–742. [Google Scholar] [CrossRef]



| All Patients | Survivors | Non-Survivors | ||||
|---|---|---|---|---|---|---|
| n = 682 | n = 451 | n = 231 | p | HR (95% CI) | p (for HR) | |
| General characteristics and comorbidities | ||||||
| Female Sex | 269 (39.2) | 182 (40.4) | 87 (37.7) | 0.157 | 0.904 (0.69–1.18) | 0.460 |
| Age | 64.1 (12) | 61.1 (11.8) | 70.2 (10) | <0.001 | 1.069 (1.05–1.08) | <0.001 |
| Hypertension | 181 (26.3) | 148 (32.8) | 201 (87) | <0.001 | 2.621 (1.79–3.85) | <0.001 |
| BMI | 27.8 (4.8) | 27.5 (4.6) | 28.6 (5.0) | 0.003 | 1.033 (1.01–1.06) | 0.010 |
| Diabetes | 231 (33.6) | 65 (14.4) | 59 (25.5) | 0.001 | 1.854 (1.38–2.49) | <0.001 |
| Coronary artery disease | 282 (41) | 154 (34.1) | 127 (55) | <0.001 | 1.992 (1.54–2.58) | <0.001 |
| Atrial fibrillation | 180 (26.2) | 84 (18.6) | 96 (41.6) | <0.001 | 2.486 (1.91–3.23) | <0.001 |
| NYHA class | 2.53 (0.58) | 2.50 (0.59) | 2.58 (0.57) | <0.05 | 1.258 (1.006–1.574) | 0.044 |
| Serum parameters | ||||||
| Creatinine (mg/dL) | 1.03 (0.4) | 0.97 (0.27) | 1.15 (0.47) | <0.001 | 2.352 (1.92–2.88) | <0.001 |
| Hemoglobin (g/dL) | 13.9 (1.5) | 14.0 (1.4) | 13.6 (1.7) | 0.001 | 0.844 (0.78–0.92) | <0.001 |
| Sodium (mmol/L) | 140 (3.2) | 140 (2.9) | 139 (3.7) | 0.032 | 0.961 (0.92–1.00) | 0.058 |
| Echocardiography | ||||||
| Left atrial diameter (mm) | 44 (8) | 43 (7) | 46 (8) | <0.001 | 1.045 (1.03–1.06) | <0.001 |
| EFTeichholz (%) | 59 (16) | 60 (16) | 58 (17) | 0.186 | 0.995 (0.99–1.00) | 0.231 |
| LVEDD (mm) | 54 (9) | 55 (9) | 54 (8) | 0.506 | 0.993 (0.98–1.01) | 0.365 |
| LVESD (mm) | 37 (10) | 37 (10) | 37 (10) | 0.731 | 1.000 (0.99–1.01) | 0.979 |
| Medication use (n) | ||||||
| Beta-blocker | 315 (45.9) | 183 (40.6) | 131 (56.7) | <0.001 | 1.519 (1.17–1.97) | 0.002 |
| ACE-inhibitors | 257 (37.4) | 156 (34.6) | 101 (43.7) | 0.015 | 1.187 (0.92–1.54) | 0.198 |
| AT2-blockers | 127 (18.5) | 74 (16.4) | 51 (22.1) | 0.090 | 1.355 (0.99–1.85) | 0.063 |
| Any diuretic | 289 (42.1) | 154 (34.1) | 135 (58.4) | <0.001 | 2.070 (1.59–2.69) | <0.001 |
| All Patients | Survivors | Non-Survivors | ||||
|---|---|---|---|---|---|---|
| n = 682 | n = 451 | n = 231 | p | HR (95% CI) | p (for HR) | |
| Peak workload (W) | 64.2 (38.3) | 72.5 (38.5) | 46.3 (30.1) | <0.001 | 0.980 (0.98–0.99) | <0.001 |
| Peak workload/body weight (W/kg) | 0.82 (0.48) | 0.91 (0.49) | 0.57 (0.35) | <0.001 | 0.133 (0.13–0.28) | <0.001 |
| Right atrial pressure (mmHg) | ||||||
| at rest | 6.6 (4.03) | 6.00 (3.6) | 7.9 (4.6) | <0.001 | 1.073 (1.05–1.10) | <0.001 |
| at peak workload | 15.1 (6.6) | 13.9 (6.3) | 18.0 (6.3) | <0.001 | 1.069 (1.05–1.09) | <0.001 |
| Pulmonary capillary wedge pressure (mmHg) | ||||||
| at rest | 12.0 (8.0–18.0) | 10.0 (8.0–15.0) | 14.1 (10.0–21.0) | <0.001 | 1.049 (1.03–1.07) | <0.001 |
| at peak workload | 28.0 (20.0–34.0) | 27.0 (20.0–33.0) | 29.0 (23.0–35.0) | <0.001 | 1.025 (1.01–1.04) | 0.001 |
| Pulmonary artery pressure (mmHg) | ||||||
| at rest | 23.1 (9.6) | 21.2 (8.7) | 27.0 (10.5) | <0.001 | 1.040 (1.03–1.05) | <0.001 |
| at peak workload | 43.4 (10.9) | 42.1 (10.6) | 46.5 (10.6) | <0.001 | 1.025 (1.01–1.04) | <0.001 |
| Preserved EF (n = 512) | Impaired EF (<50%, Includes Both HFmrEF and HFrEF, n = 170) | |||||||
|---|---|---|---|---|---|---|---|---|
| Survivors | Non-Survivors | Survivors | Non-Survivors | |||||
| n = 342 | n = 170 | HR (95% CI) | p (for HR) | n = 109 | n = 61 | HR (95% CI) | p (for HR) | |
| Female Sex | 153 (44.7) | 68 (40.0) | 0.832 (0.61–1.13) | 0.241 | 29 (26.6) | 19 (31.1) | 0.867 (0.50–1.49) | 0.605 |
| Age | 62.2 (11.8) | 71.4 (9.8) | 1.073 (1.06–1.09) | <0.001 | 57.5 (11.2) | 66.8 (10.0) | 1.046 (1.05–1.10) | <0.001 |
| Hypertension | 227 (66.4) | 148 (87.1) | 2.706 (1.73–4.24) | <0.001 | 76 (69.7) | 53 (86.9) | 2.255 (1.07–4.74) | 0.032 |
| BMI | 27.0 (4.4) | 28.6 (5.1) | 1.050 (1.02–1.08) | <0.001 | 28.9 (4.8) | 28.5 (4.9) | 0.980 (0.93–1.04) | 0.470 |
| Diabetes | 41 (12.0) | 43 (25.3) | 1.952 (1.38–2.76) | <0.001 | 24 (22. 0) | 16 (26.2) | 1.437 (0.81–2.55) | 0.214 |
| Coronary artery disease | 112 (32.7) | 92 (54.1) | 1.913 (1.42–2.59) | <0.001 | 42 (38.5) | 35 (57.3) | 2.069 (1.24–3.45) | 0.005 |
| Atrial fibrillation | 64 (18.7) | 72 (42.4) | 2.497 (1.84–3.39) | <0.001 | 20 (18.3) | 24 (39.3) | 2.351 (1.40–3.94) | 0.001 |
| Creatine (mg/dL) | 0.95 (0.25) | 1.13 (0.49) | 2.340 (1.85–2.95) | <0.001 | 1.04 (0.3) | 1.20 (0.4) | 3.105 (1.70–5.68) | <0.001 |
| Hemoglobin (g/dL) | 14.0 (1.3) | 13.6 (1.3) | 0.848 (0.77–0.94) | 0.002 | 14.3 (1.8) | 13.9 (1.5) | 0.844 (0.73–0.97) | 0.020 |
| Sodium (mmol/L) | 140 (3.0) | 140 (3.9) | 0.976 (0.93–1.02) | 0.309 | 140 (2.6) | 139 (3.3) | 0.893 (0.82–0.98) | 0.015 |
| Left atrial diameter (mm) | 43 (7) | 45 (9) | 1.049 (1.03–1.07) | <0.001 | 44 (9) | 48 (9) | 1.036 (1.01–1.07) | 0.020 |
| EFTeichholz | 67 (9) | 66 (9) | 0.992 (0.98–1.01) | 0.396 | 37 (10) | 35 (11) | 0.989 (0.96–1.01) | 0.372 |
| LVEDD | 53 (7) | 52 (7) | 0.997 (0.98–1.02) | 0.788 | 61 (11) | 59 (10) | 0.981 (0.96–1.01) | 0.130 |
| LVESD | 33 (6) | 33 (6) | 1.003 (0.98–1.03) | 0.835 | 50 (10) | 49 (10) | 0.986 (0.96–1.01) | 0.299 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahlgrim, C.; Kocher, S.; Minners, J.; Jander, N.; Savarese, G.; Neumann, F.-J.; Arentz, T.; Jadidi, A.; Mueller-Edenborn, B. Pulmonary Capillary Wedge Pressure during Exercise Is Prognostic for Long-Term Survival in Patients with Symptomatic Heart Failure. J. Clin. Med. 2022, 11, 5901. https://doi.org/10.3390/jcm11195901
Ahlgrim C, Kocher S, Minners J, Jander N, Savarese G, Neumann F-J, Arentz T, Jadidi A, Mueller-Edenborn B. Pulmonary Capillary Wedge Pressure during Exercise Is Prognostic for Long-Term Survival in Patients with Symptomatic Heart Failure. Journal of Clinical Medicine. 2022; 11(19):5901. https://doi.org/10.3390/jcm11195901
Chicago/Turabian StyleAhlgrim, Christoph, Sascha Kocher, Jan Minners, Nikolaus Jander, Gianluigi Savarese, Franz-Josef Neumann, Thomas Arentz, Amir Jadidi, and Björn Mueller-Edenborn. 2022. "Pulmonary Capillary Wedge Pressure during Exercise Is Prognostic for Long-Term Survival in Patients with Symptomatic Heart Failure" Journal of Clinical Medicine 11, no. 19: 5901. https://doi.org/10.3390/jcm11195901
APA StyleAhlgrim, C., Kocher, S., Minners, J., Jander, N., Savarese, G., Neumann, F.-J., Arentz, T., Jadidi, A., & Mueller-Edenborn, B. (2022). Pulmonary Capillary Wedge Pressure during Exercise Is Prognostic for Long-Term Survival in Patients with Symptomatic Heart Failure. Journal of Clinical Medicine, 11(19), 5901. https://doi.org/10.3390/jcm11195901







