Association of the Posterior Acromion Extension with Glenoid Retroversion: A CT Study in Normal and Osteoarthritic Shoulders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Protocols
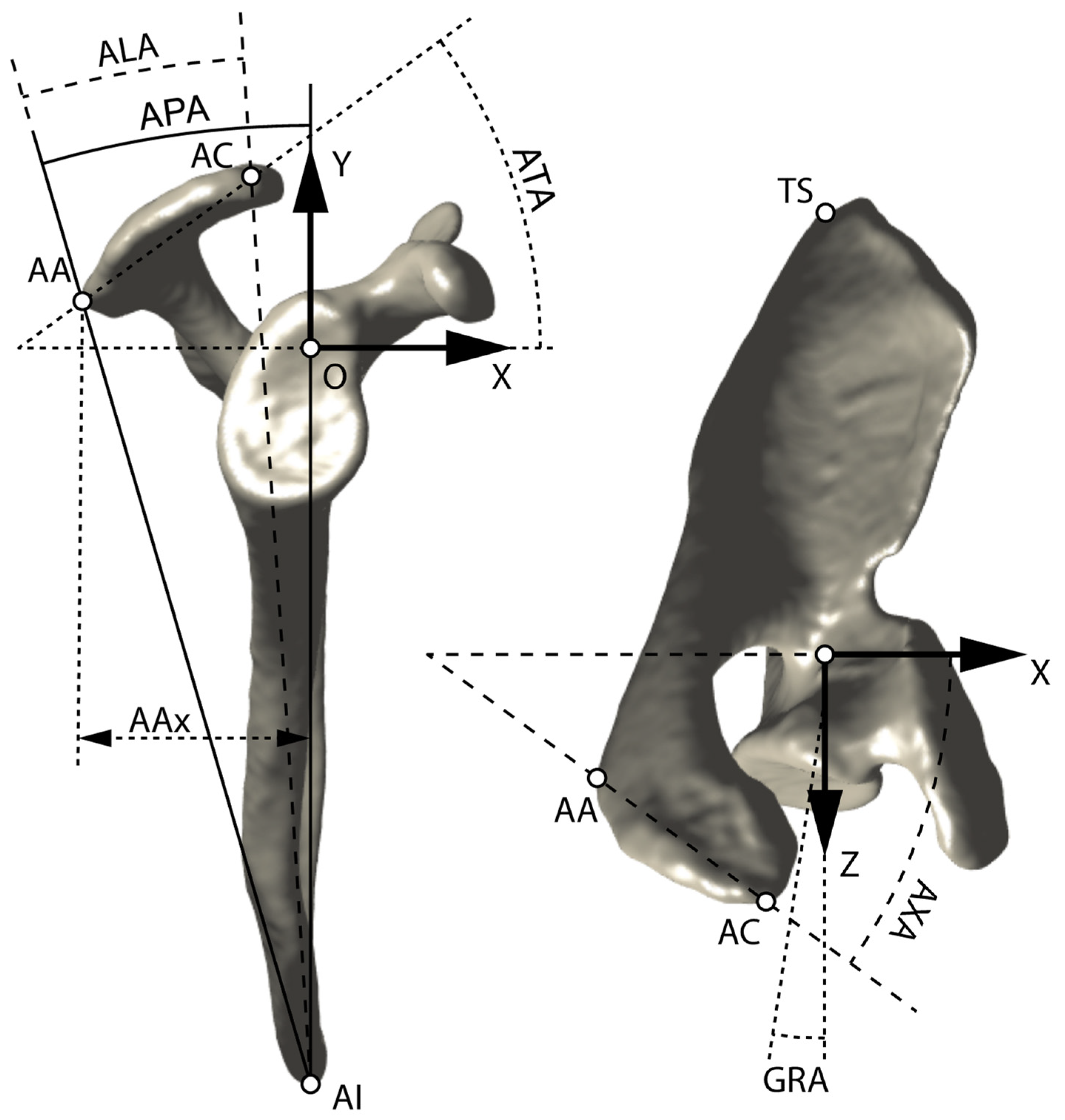
2.3. Scapular Coordinate System
2.4. Acromion Landmarks
2.5. Acromion Angles
2.6. Glenoid Retroversion Angle
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyffeler, R.W.; Meyer, D.C. Acromion and Glenoid Shape: Why Are They Important Predictive Factors for the Future of Our Shoulders? EFORT Open Rev. 2017, 2, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyffeler, R.W.; Werner, C.M.L.; Sukthankar, A.; Schmid, M.R.; Gerber, C. Association of a Large Lateral Extension of the Acromion with Rotator Cuff Tears. J. Bone Jt. Surg. Am. 2006, 88, 800–805. [Google Scholar] [CrossRef]
- Moor, B.K.; Bouaicha, S.; Rothenfluh, D.A.; Sukthankar, A.; Gerber, C. Is There an Association between the Individual Anatomy of the Scapula and the Development of Rotator Cuff Tears or Osteoarthritis of the Glenohumeral Joint?: A Radiological Study of the Critical Shoulder Angle. Bone Jt. J. 2013, 95-B, 935–941. [Google Scholar] [CrossRef]
- Engelhardt, C.; Farron, A.; Becce, F.; Place, N.; Pioletti, D.P.; Terrier, A. Effects of Glenoid Inclination and Acromion Index on Humeral Head Translation and Glenoid Articular Cartilage Strain. J. Shoulder Elb. Surg. 2017, 26, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Viehöfer, A.F.; Snedeker, J.G.; Baumgartner, D.; Gerber, C. Glenohumeral Joint Reaction Forces Increase with Critical Shoulder Angles Representative of Osteoarthritis-A Biomechanical Analysis. J. Orthop. Res. 2016, 34, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Jacxsens, M.; Van Tongel, A.; Henninger, H.B.; De Coninck, B.; Mueller, A.M.; De Wilde, L. A Three-Dimensional Comparative Study on the Scapulohumeral Relationship in Normal and Osteoarthritic Shoulders. J. Shoulder Elb. Surg. 2016, 25, 1607–1615. [Google Scholar] [CrossRef]
- Beeler, S.; Hasler, A.; Getzmann, J.; Weigelt, L.; Meyer, D.C.; Gerber, C. Acromial Roof in Patients with Concentric Osteoarthritis and Massive Rotator Cuff Tears: Multiplanar Analysis of 115 Computed Tomography Scans. J. Shoulder Elb. Surg. 2018, 27, 1866–1876. [Google Scholar] [CrossRef]
- Beeler, S.; Hasler, A.; Götschi, T.; Meyer, D.C.; Gerber, C. Different Acromial Roof Morphology in Concentric and Eccentric Osteoarthritis of the Shoulder: A Multiplane Reconstruction Analysis of 105 Shoulder Computed Tomography Scans. J. Shoulder Elb. Surg. 2018, 27, e357–e366. [Google Scholar] [CrossRef]
- Meyer, D.C.; Riedo, S.; Eckers, F.; Carpeggiani, G.; Jentzsch, T.; Gerber, C. Small Anteroposterior Inclination of the Acromion Is a Predictor for Posterior Glenohumeral Erosion (B2 or C). J. Shoulder Elb. Surg. 2019, 28, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Beeler, S.; Leoty, L.; Hochreiter, B.; Carrillo, F.; Götschi, T.; Fischer, T.; Fürnstahl, P.; Gerber, C. Similar Scapular Morphology in Patients with Dynamic and Static Posterior Shoulder Instability. JSES Int. 2021, 5, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Walch, G.; Ascani, C.; Boulahia, A.; Nové-Josserand, L.; Edwards, T.B. Static Posterior Subluxation of the Humeral Head: An Unrecognized Entity Responsible for Glenohumeral Osteoarthritis in the Young Adult. J. Shoulder Elb. Surg. 2002, 11, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Walch, G.; Moraga, C.; Young, A.; Castellanos-Rosas, J. Results of Anatomic Nonconstrained Prosthesis in Primary Osteoarthritis with Biconcave Glenoid. J. Shoulder Elb. Surg. 2012, 21, 1526–1533. [Google Scholar] [CrossRef]
- Domos, P.; Checchia, C.S.; Walch, G. Walch B0 Glenoid: Pre-Osteoarthritic Posterior Subluxation of the Humeral Head. J. Shoulder Elb. Surg. 2018, 27, 181–188. [Google Scholar] [CrossRef]
- Sabesan, V.J.; Callanan, M.; Youderian, A.; Iannotti, J.P. 3D CT Assessment of the Relationship between Humeral Head Alignment and Glenoid Retroversion in Glenohumeral Osteoarthritis. J. Bone Jt. Surg. Am. 2014, 96, e64. [Google Scholar] [CrossRef]
- Gerber, C.; Costouros, J.G.; Sukthankar, A.; Fucentese, S.F. Static Posterior Humeral Head Subluxation and Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2009, 18, 505–510. [Google Scholar] [CrossRef]
- Zember, J.S.; Rosenberg, Z.S.; Kwong, S.; Kothary, S.P.; Bedoya, M.A. Normal Skeletal Maturation and Imaging Pitfalls in the Pediatric Shoulder. RadioGraphics 2015, 35, 1108–1122. [Google Scholar] [CrossRef]
- Bercik, M.J.; Kruse, K.; Yalizis, M.; Gauci, M.-O.; Chaoui, J.; Walch, G. A Modification to the Walch Classification of the Glenoid in Primary Glenohumeral Osteoarthritis Using Three-Dimensional Imaging. J. Shoulder Elb. Surg. 2016, 25, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Terrier, A.; Ston, J.; Farron, A. Importance of a Three-Dimensional Measure of Humeral Head Subluxation in Osteoarthritic Shoulders. J. Shoulder Elb. Surg. 2015, 24, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrier, A.; Ston, J.; Larrea, X.; Farron, A. Measurements of Three-Dimensional Glenoid Erosion When Planning the Prosthetic Replacement of Osteoarthritic Shoulders. Bone Jt. J. 2014, 96-B, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Luedke, C.; Kissenberth, M.J.; Tolan, S.J.; Hawkins, R.J.; Tokish, J.M. Outcomes of Anatomic Total Shoulder Arthroplasty with B2 Glenoids: A Systematic Review. JBJS Rev. 2018, 6, e7. [Google Scholar] [CrossRef]
- Shapiro, T.A.; McGarry, M.H.; Gupta, R.; Lee, Y.S.; Lee, T.Q. Biomechanical Effects of Glenoid Retroversion in Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2007, 16, S90–S95. [Google Scholar] [CrossRef]
- Logli, A.L.; Pareek, A.; Nguyen, N.T.V.; Sanchez-Sotelo, J. Natural History of Glenoid Bone Loss in Primary Glenohumeral Osteoarthritis: How Does Bone Loss Progress over a Decade? J. Shoulder Elb. Surg. 2021, 30, 324–330. [Google Scholar] [CrossRef]
- Kothary, P.; Rosenberg, Z.S. Skeletal Developmental Patterns in the Acromial Process and Distal Clavicle as Observed by MRI. Skelet. Radiol. 2015, 44, 207–215. [Google Scholar] [CrossRef]
- Knowles, N.K.; Ferreira, L.M.; Athwal, G.S. Premorbid Retroversion Is Significantly Greater in Type B2 Glenoids. J. Shoulder Elb. Surg. 2016, 25, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.W.; Chalmers, P.N.; Bechtold, D.; Khan, A.Z.; Tashjian, R.Z.; Keener, J.D. Association Between Rotator Cuff Muscle Size and Glenoid Deformity in Primary Glenohumeral Osteoarthritis. J. Bone Jt. Surg. Am. 2019, 101, 1912–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raniga, S.; Knowles, N.K.; West, E.; Ferreira, L.M.; Athwal, G.S. The Walch Type B Humerus: Glenoid Retroversion Is Associated with Torsional Differences in the Humerus. J. Shoulder Elb. Surg. 2019, 28, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Voisin, J.-L.; Ropars, M.; Thomazeau, H. The Human Acromion Viewed from an Evolutionary Perspective. Orthop. Traumatol. Surg. Res. 2014, 100, S355–S360. [Google Scholar] [CrossRef] [Green Version]
- Kitay, G.S.; Iannotti, J.P.; Williams, G.R.; Haygood, T.; Kneeland, B.J.; Berlin, J. Roentgenographic Assessment of Acromial Morphologic Condition in Rotator Cuff Impingement Syndrome. J. Shoulder Elb. Surg. 1995, 4, 441–448. [Google Scholar] [CrossRef]
- Taghizadeh, E.; Terrier, A.; Becce, F.; Farron, A.; Büchler, P. Automated CT Bone Segmentation Using Statistical Shape Modelling and Local Template Matching. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 1303–1310. [Google Scholar] [CrossRef]


| RMSE (Degree) | R2 | p-Value | |
|---|---|---|---|
| AAx | 3.73 | 0.480 | <0.0001 |
| AAy | 5.16 | 0.006 | 0.4308 |
| AAz | 5.02 | 0.051 | 0.0096 |
| ACx | 4.31 | 0.310 | <0.0001 |
| Acy | 5.14 | 0.013 | 0.2298 |
| ACz | 5.16 | 0.007 | 0.3739 |
| AAx, AAz | 3.66 | 0.505 | <0.0001 |
| AAx, AAz, ACx | 3.58 | 0.530 | <0.0001 |
| APA | 3.73 | 0.482 | <0.0001 |
| ALA | 5.17 | 0.002 | 0.6305 |
| ATA | 5.12 | 0.022 | 0.1187 |
| AXA | 4.85 | 0.123 | 0.0001 |
| APA, ALA | 3.61 | 0.518 | <0.0001 |
| APA, ALA, ATA | 3.50 | 0.551 | <0.0001 |
| Scapulae | GRA (Degree) | APA (Degree) |
|---|---|---|
| Normal (n = 112) | 3.0 ± 5.2 | 14.9 ± 1.9 |
| Walch type A1–A2 (n = 49) | 4.5 ± 9.4 | 15.3 ± 2.3 |
| Walch type B1 (n = 26) | 10.1 ± 9.0 | 15.2 ± 1.8 |
| Walch type B2–B3 (n = 45) | 16.9 ± 8.1 | 16.3 ± 2.2 |
| Walch type C (n = 5) | 29.6 ± 8.4 | 16.9 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrier, A.; Becce, F.; Vauclair, F.; Farron, A.; Goetti, P. Association of the Posterior Acromion Extension with Glenoid Retroversion: A CT Study in Normal and Osteoarthritic Shoulders. J. Clin. Med. 2022, 11, 351. https://doi.org/10.3390/jcm11020351
Terrier A, Becce F, Vauclair F, Farron A, Goetti P. Association of the Posterior Acromion Extension with Glenoid Retroversion: A CT Study in Normal and Osteoarthritic Shoulders. Journal of Clinical Medicine. 2022; 11(2):351. https://doi.org/10.3390/jcm11020351
Chicago/Turabian StyleTerrier, Alexandre, Fabio Becce, Frédéric Vauclair, Alain Farron, and Patrick Goetti. 2022. "Association of the Posterior Acromion Extension with Glenoid Retroversion: A CT Study in Normal and Osteoarthritic Shoulders" Journal of Clinical Medicine 11, no. 2: 351. https://doi.org/10.3390/jcm11020351
APA StyleTerrier, A., Becce, F., Vauclair, F., Farron, A., & Goetti, P. (2022). Association of the Posterior Acromion Extension with Glenoid Retroversion: A CT Study in Normal and Osteoarthritic Shoulders. Journal of Clinical Medicine, 11(2), 351. https://doi.org/10.3390/jcm11020351







