A Tear Metabolomic Profile Showing Increased Ornithine Decarboxylase Activity and Spermine Synthesis in Thyroid-Associated Orbitopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Tear Sampling and Metabolite Extraction
2.3. Metabolite Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Description
3.2. Altered Metabolite Concentrations
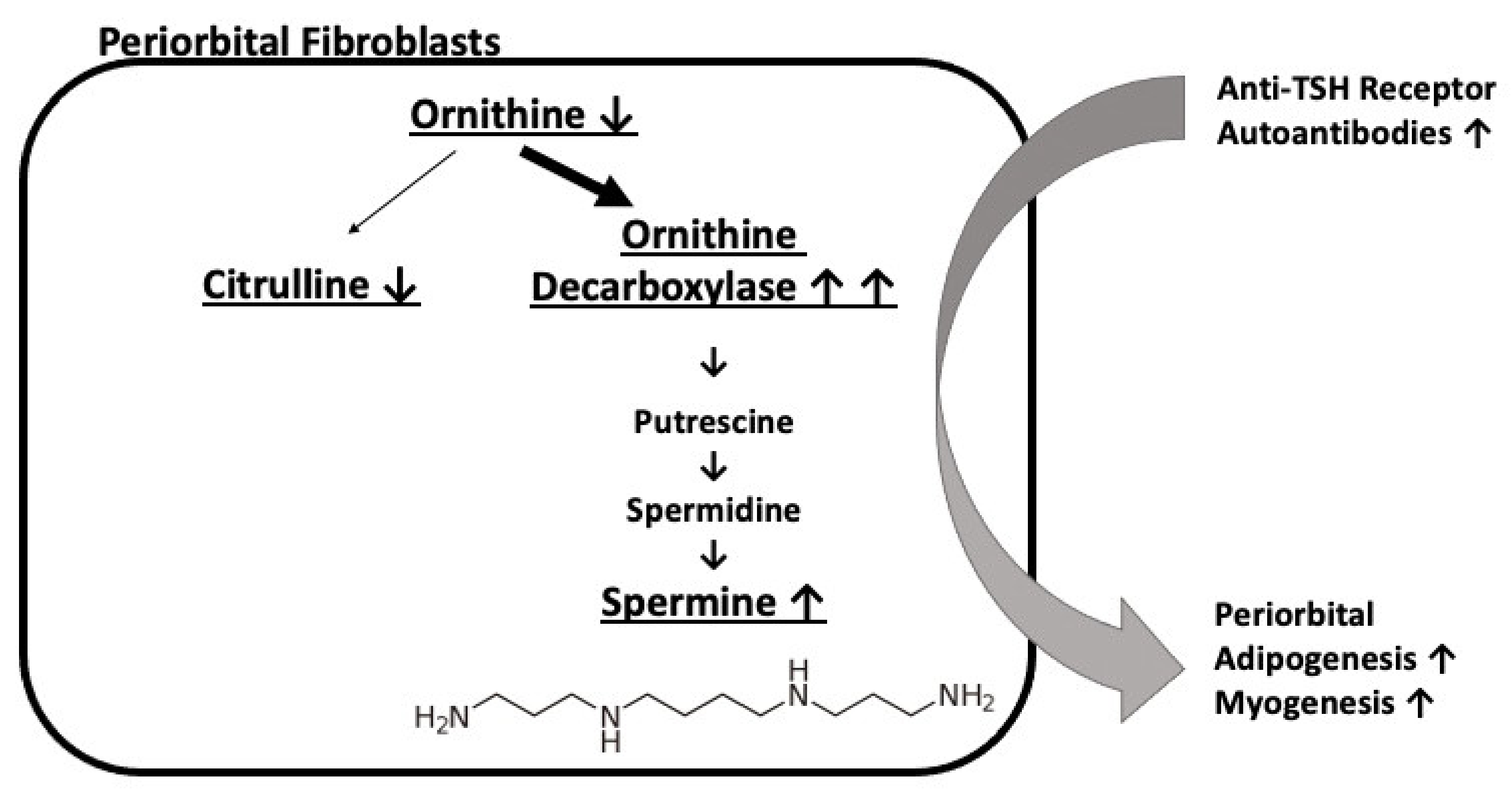
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Perros, P.; Hegedüs, L.; Bartalena, L.; Marcocci, C.; Kahaly, G.J.; Baldeschi, L.; Salvi, M.; Lazarus, J.H.; Eckstein, A.; Pitz, S.; et al. Graves’ orbitopathy as a rare disease in Europe: A European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J. Rare Dis. 2017, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S. Graves’ ophthalmopathy. N. Engl. J. Med. 2010, 362, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Potential role for bone marrow-derived fibrocytes in the orbital fibroblast heterogeneity associated with thyroid-associated ophthalmopathy. Clin. Exp. Immunol. 2010, 162, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.M.; Higgins, P.J. Molecular biomarkers of Graves’ ophthalmopathy. Exp. Mol. Pathol. 2019, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bartley, G.B.; Fatourechi, V.; Kadrmas, E.F.; Ilstrup, D.M.; Garrity, J.A.; Gorman, C.A. Chronology of Graves’ ophthalmopathy in an incidence cohort. Am. J. Ophthalmol. 1996, 121, 426–434. [Google Scholar] [CrossRef]
- Prummel, M.F.; Wiersinga, W.M. Smoking and risk of Graves’ disease. JAMA 1993, 269, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marino, M.; Vaidya, B.; Wiersinga, W.M.; EUGOGO. The 2021 European group on Graves’ orbitopathy (EUGOGO) clinical guidelines for the medical management of Graves’s orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef] [PubMed]
- Mourits, M.P.; Prummel, M.F.; Wiersinga, W.M.; Koornneef, L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin. Endocrinol. 1997, 47, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Martin, E.; Enríquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Elgstøen, K.B.P.; Rootwelt, H.; Shahdadfar, A.; Utheim, Ø.A.; Utheim, T.P. Tear Metabolomics in Dry Eye Disease: A Review. Int. J. Mol. Sci. 2019, 20, 3755. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, G.; Assad, S.; Chabrun, F.; Chao de la Barca, J.M.; Blanchet, O.; Simard, G.; Lenaers, G.; Prunier-Mirebeau, D.; Gohier, P.; Lavigne, C.; et al. Tear metabolomics highlights new potential biomarkers for differentiating between Sjögren’s syndrome and other causes of dry eye. Ocul. Surf. 2021, 22, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.Y.; Park, S.H.; Park, S.J.; Kim, K.H.; Ku, C.R.; Shin, D.Y.; Yoon, J.S.; Lee, D.Y.; Lee, E.J. Comparative assessment of Graves’ disease and main extrathyroidal manifestation, Graves’ ophthalmopathy, by non-targeted metabolite profiling of blood and orbital tissue. Sci. Rep. 2018, 8, 9262. [Google Scholar] [CrossRef] [PubMed]
- Al-Majdoub, M.; Lantz, M.; Spégel, P. Treatment of Swedish Patients with Graves’ Hyperthyroidism Is Associated with Changes in Acylcarnitine Levels. Thyroid 2017, 27, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Richman, R.; Park, S.; Akbar, M.; Yu, S.; Burke, G. Regulation of thyroid ornithine decarboxylase (ODC) by thyrotropin. I. The rat. Endocrinology 1975, 96, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Zusman, D.R.; Burrow, G.N. Thyroid-stimulating hormone regulation of ornithine decarboxylase activity in the thyroid. Endocrinology 1975, 97, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S. A potential role of polyamines in goiter formation (author’s translation). Nihon Naibunpi Gakkai Zasshi 1981, 57, 1554–1566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, H.T.; Reuse, S.; Koibuchi, N.; Matsuzaki, S. Acute inhibitory effect of excess iodide on ornithine decarboxylase in the thyroid of propylthiouracil-treated rats. J. Endocrinol. 1996, 150, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; MacLean, H.E. Polyamines, androgens, and skeletal muscle hypertrophy. J. Cell. Physiol. 2011, 226, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Ikeguchi, Y.; Mano, H.; Wada, M.; Pegg, A.E.; Shirahata, A. Polyamine metabolism is involved in adipogenesis of 3T3-L1 cells. Amino Acids 2012, 42, 619–626. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Clinical Data | Active (n = 21) | Inactive (n = 24) | p-Values |
|---|---|---|---|
| Demographic data | |||
| Women (%) | 76% (16/21) | 75% (18/24) | 0.93 |
| Mean age (years) | 55 | 51 | 0.42 |
| Past smoker (%) | 42% (5/12) | 48% (10/21) | 0.74 |
| Active smoker (%) | 0% (0/12) | 33% (7/21) | 0.02 |
| Diabetes (%) | 10% (2/21) | 4% (1/24) | 0.47 |
| Hypertension (%) | 19% (4/21) | 13% (3/24) | 0.55 |
| Hyperlipidemia (%) | 5% (1/21) | 4% (1/24) | 0.92 |
| Graves’ disease features | |||
| TRAb positivity (%) | 95% (19/20) | 76% (13/17) | 0.10 |
| Mean TSH (mUI/L) | 3.43 | 2.84 | 0.75 |
| Mean fT4 (pmol) | 12.35 | 15.44 | 0.28 |
| Radiotherapy (%) | 14% (3/21) | 17% (4/24) | 0.83 |
| Corticosteroid (%) | 38% (8/21) | 37% (9/24) | 0.97 |
| Thyroidectomy (%) | 19% (4/21) | 25% (6/24) | 0.63 |
| Duration of dysthyroidism (months) | 41 | 50 | 0.64 |
| Antithyroid drugs (%) | 75% (15/20) | 54% (13/24) | 0.15 |
| Thyroid hormones (%) | 55% (11/20) | 71% (17/24) | 0.28 |
| Ophthalmological features | |||
| Clinical Activity Score | 3.67 | 0.82 | <0.001 |
| Oxford score | 0.43 | 0.08 | 0.01 |
| Mean visual acuity (log mar) | 0.079 | 0.065 | 0.68 |
| Mean Schirmer imbibition (mm) | 35 | 32 | 0.19 |
| Metabolites | p-Values | Fold Changes |
|---|---|---|
| Spermine | 0.0074 | 1.34 |
| Butyrylcarnitine (C4) | 0.0176 | 1.54 |
| Ornithine | 0.0189 | 0.43 |
| Propionylcarnitine (C3) | 0.0232 | 1.77 |
| Glycine | 0.0235 | 0.45 |
| Serine | 0.0278 | 0.38 |
| Citrulline | 0.0281 | 0.23 |
| Histidine | 0.0336 | 0.45 |
| Ratio | ||
| Putrescine/Ornithine (ornithine decarboxylase activity) | 0.0011 | 3.75 |
| Demographic and Clinical Data | Controls (n = 20) |
|---|---|
| Women (%) | 80% |
| Mean age (years) | 55 |
| Mean Schirmer imbibition (mm) | 34.6 |
| Past smoker (%) | 36% |
| Active smoker (%) | 27% |
| Diabetes (%) | 25% |
| Hypertension (%) | 25% |
| Hyperlipidemia (%) | 0% |
| TRAb positivity (%) | NA |
| Mean TSH (mUI/L) | NA |
| Mean fT4 (pmol) | NA |
| Radiotherapy (%) | NA |
| Corticosteroid (%) | NA |
| Thyroidectomy (%) | NA |
| Duration of dysthyroidism (months) | NA |
| Oxford score | 0.15 |
| Mean visual acuity (log mar) | 0.1475 |
| Clinical Activity Score | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billiet, B.; Chao de la Barca, J.M.; Ferré, M.; Muller, J.; Vautier, A.; Assad, S.; Blanchet, O.; Tessier, L.; Wetterwald, C.; Faure, J.; et al. A Tear Metabolomic Profile Showing Increased Ornithine Decarboxylase Activity and Spermine Synthesis in Thyroid-Associated Orbitopathy. J. Clin. Med. 2022, 11, 404. https://doi.org/10.3390/jcm11020404
Billiet B, Chao de la Barca JM, Ferré M, Muller J, Vautier A, Assad S, Blanchet O, Tessier L, Wetterwald C, Faure J, et al. A Tear Metabolomic Profile Showing Increased Ornithine Decarboxylase Activity and Spermine Synthesis in Thyroid-Associated Orbitopathy. Journal of Clinical Medicine. 2022; 11(2):404. https://doi.org/10.3390/jcm11020404
Chicago/Turabian StyleBilliet, Benjamin, Juan Manuel Chao de la Barca, Marc Ferré, Jeanne Muller, Anaïs Vautier, Sophie Assad, Odile Blanchet, Lydie Tessier, Céline Wetterwald, Justine Faure, and et al. 2022. "A Tear Metabolomic Profile Showing Increased Ornithine Decarboxylase Activity and Spermine Synthesis in Thyroid-Associated Orbitopathy" Journal of Clinical Medicine 11, no. 2: 404. https://doi.org/10.3390/jcm11020404
APA StyleBilliet, B., Chao de la Barca, J. M., Ferré, M., Muller, J., Vautier, A., Assad, S., Blanchet, O., Tessier, L., Wetterwald, C., Faure, J., Urbanski, G., Simard, G., Mirebeau-Prunier, D., Rodien, P., Gohier, P., & Reynier, P. (2022). A Tear Metabolomic Profile Showing Increased Ornithine Decarboxylase Activity and Spermine Synthesis in Thyroid-Associated Orbitopathy. Journal of Clinical Medicine, 11(2), 404. https://doi.org/10.3390/jcm11020404






