A Narrative Review of the Association between Post-Traumatic Stress Disorder and Obstructive Sleep Apnea
Abstract
:1. Introduction
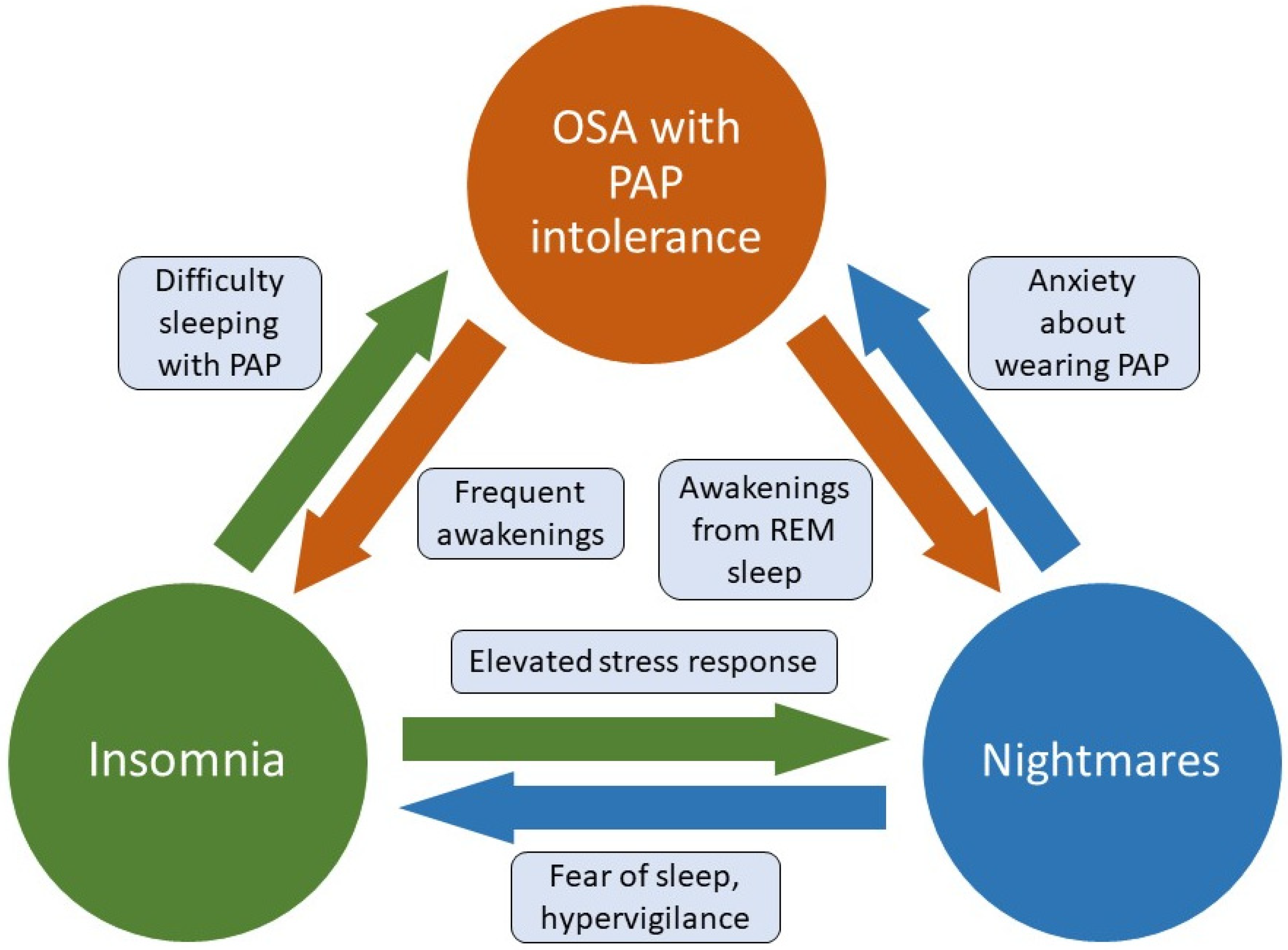
2. The OSA and PTSD Overlap
3. Impacts of OSA Treatment on PTSD
3.1. PAP Therapy
3.2. Alternative OSA Treatments
| Authors | Year | Study Type | Study Population | Age (Mean Years ± SD) | Sex (% Male) | Treatment Type | Main Findings |
|---|---|---|---|---|---|---|---|
| Youakim et al. [81] | 1998 | Case Report | Veteran | 42 | 100 | PAP therapy | Nightmare frequency and intensity was improved after 4 months of PAP therapy, as well as daytime PTSD symptoms. |
| Krakow et al. [82] | 2000 | Retrospective | Civilians | Treatment: 43.8 ± 14.1 No treatment: 50.8 ± 14.9 | Not reported | PAP therapy | PAP users reported a median 75% improvement in PTSD symptoms; subjects without PAP therapy reported worsening symptoms. |
| Tamanna et al. [83] | 2014 | Retrospective | Veterans | 58 ± 12.05 | 97 | PAP therapy | The mean number of nightmares per week was reduced over 6 months of PAP therapy. Reduced nightmare frequency was best predicted by PAP adherence. |
| El-Solh et al. [84] | 2017a | Prospective cohort | Veterans | 52.6 ± 14.2 | 92.5 | PAP therapy | PCL-M scores improved after 3 months of PAP therapy, in a dose-dependent manner. PAP usage was the only significant predictor of overall PTSD symptom improvement. |
| Orr et al. [85] | 2017 | Prospective cohort | Veterans | 52 (range 43-65) | 87.5 | PAP therapy | PCL-S scores improved over 6 months of PAP therapy. The percentage of nights in which PAP was used, but not mean hours used per night, predicted improvement. |
| Ullah et al. [86] | 2017 | Prospective cohort | Veterans | 51.24 ± 14.74 | Not reported | PAP therapy | PCL-M scores improved after 6 months of PAP therapy in PTSD patients, whereas non-PTSD patients with low adherence showed worsening of PCL-M scores. |
| El-Solh et al. [89] | 2017b | Randomized crossover trial | Veterans | 52.7 ± 11.6 | Not reported | MRD compared to PAP therapy | 71% of CPAP users and 14% of MRD users had complete OSA resolution during titration studies; however MRD users had longer sleep time, higher sleep efficiency and better adherence to treatment. Both treatments showed similar improvements in PCL-M scores after 3 months. |
| El-Solh et al. [90] | 2018 | Prospective | Veterans | PTSD with comorbid OSA and insomnia: 47.2 ± 10.8 | PTSD with comorbid OSA and insomnia: 72 | PAP therapy | PCL-M scores improved after 3 months of PAP therapy in patients with and without insomnia. The change in PCL-M scores was smaller in those with insomnia. PAP adherence was also lower in the insomnia group. |
| PTSD with OSA: 52.7 ± 9.7 | PTSD with OSA: 86 | ||||||
| Patil et al. [97] | 2021 | Retrospective and prospective case series | Veterans | 59.3 ± 10.6 | 96.2 | HNS | Resolution of OSA and adherence were similar for patients with and without PTSD; adherence was lower in PTSD patients with insomnia. PCL-5 scores obtained 6–12 months after surgery did not significantly change from baseline. |
4. Conclusions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Babson, K.A.; Feldner, M.T. Temporal relations between sleep problems and both traumatic event exposure and PTSD: A critical review of the empirical literature. J. Anxiety Disord. 2010, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, D.G.; Resnick, H.S.; Milanak, M.E.; Miller, M.; Keyes, K.M.; Friedman, M.J. National Estimates of Exposure to Traumatic Events and PTSD Prevalence UsingDSM-IVandDSM-5Criteria. J. Trauma. Stress 2013, 26, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Prim. 2015, 1, nrdp201557. [Google Scholar] [CrossRef] [PubMed]
- Rothbaum, B.O.; Foa, E.B.; Riggs, D.S.; Murdock, T.; Walsh, W. A prospective examination of post-traumatic stress disorder in rape victims. J. Trauma. Stress 1992, 5, 455–475. [Google Scholar] [CrossRef]
- Cahill, S.P.; Pontoski, K. Post-traumatic stress disorder and acute stress disorder I: Their nature and assessment considerations. Psychiatry 2005, 2, 14–25. [Google Scholar] [PubMed]
- Ohayon, M.M.; Shapiro, C.M. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr. Psychiatry 2000, 41, 469–478. [Google Scholar] [CrossRef] [PubMed]
- El-Solh, A.A.; Riaz, U.; Roberts, J. Sleep Disorders in Patients with Posttraumatic Stress Disorder. Chest 2018, 154, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Mysliwiec, V.; O’Reilly, B.; Polchinski, J.; Kwon, H.P.; Germain, A.; Roth, B.J. Trauma Associated Sleep Disorder: A Proposed Parasomnia Encompassing Disruptive Nocturnal Behaviors, Nightmares, and REM without Atonia in Trauma Survivors. J. Clin. Sleep Med. 2014, 10, 1143–1148. [Google Scholar] [CrossRef] [Green Version]
- Feemster, J.C.; Smith, K.L.; McCarter, S.J.; Louis, E.K.S. Trauma-Associated Sleep Disorder: A Posttraumatic Stress/REM Sleep Behavior Disorder Mash-Up? J. Clin. Sleep Med. 2019, 15, 345–349. [Google Scholar] [CrossRef]
- Breslau, N.; Roth, T.; Rosenthal, L.; Andreski, P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young Adults. Biol. Psychiatry 1996, 39, 411–418. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8679786 (accessed on 25 February 2018). [CrossRef]
- Ford, D.E.; Kamerow, D.B. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA 1989, 262, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Britt, T.W.; Bliese, P.D.; Adler, A.B.; Picchioni, D.; Moore, D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J. Clin. Psychol. 2011, 67, 1240–1258. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Creamer, M.; O’Donnell, M.; Silove, D.; McFarlane, A.C. Sleep Disturbance Immediately Prior to Trauma Predicts Subsequent Psychiatric Disorder. Sleep 2010, 33, 69–74. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20120622 (accessed on 2 August 2018). [CrossRef] [PubMed] [Green Version]
- Gupta, M.A.; Jarosz, P. Obstructive Sleep Apnea Severity is Directly Related to Suicidal Ideation in Posttraumatic Stress Disorder. J. Clin. Sleep Med. 2018, 14, 427–435. [Google Scholar] [CrossRef]
- Krakow, B.; Melendrez, D.; Johnston, L.; Warner, T.D.; Clark, J.O.; Pacheco, M.; Pedersen, B.; Koss, M.; Hollifield, M.; Schrader, R. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J. Nerv. Ment. Dis. 2002, 190, 442–452. [Google Scholar] [CrossRef]
- Krakow, B.; Artar, A.; Warner, T.D.; Melendrez, D.; Johnston, L.; Hollifield, M.; Germain, A.; Koss, M. Sleep Disorder, Depression, and Suicidality in Female Sexual Assault Survivors. Crisis 2000, 21, 163–170. [Google Scholar] [CrossRef]
- Walters, E.M.; Jenkins, M.M.; Nappi, C.M.; Clark, J.; Lies, J.; Norman, S.B.; Drummond, S.P.A. The impact of prolonged exposure on sleep and enhancing treatment outcomes with evidence-based sleep interventions: A pilot study. Psychol. Trauma Theory Res. Pract. Policy 2020, 12, 175–185. [Google Scholar] [CrossRef]
- Zayfert, C.; DeViva, J.C. Residual insomnia following cognitive behavioral therapy for PTSD. J. Trauma. Stress 2004, 17, 69–73. [Google Scholar] [CrossRef]
- Pruiksma, K.E.; Taylor, D.J.; Wachen, J.S.; Mintz, J.; Young-McCaughan, S.; Peterson, A.L.; Yarvis, J.S.; Borah, E.V.; Dondanville, K.A.; Litz, B.; et al. Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychol. Trauma Theory Res. Pract. Policy 2016, 8, 697–701. [Google Scholar] [CrossRef]
- Gutner, C.A.; Casement, M.; Gilbert, K.S.; Resick, P.A. Change in sleep symptoms across Cognitive Processing Therapy and Prolonged Exposure: A longitudinal perspective. Behav. Res. Ther. 2013, 51, 817–822. [Google Scholar] [CrossRef] [Green Version]
- López, C.M.; Lancaster, C.L.; Wilkerson, A.; Gros, D.F.; Ruggiero, K.J.; Acierno, R. Residual Insomnia and Nightmares Postintervention Symptom Reduction Among Veterans Receiving Treatment for Comorbid PTSD and Depressive Symptoms. Behav. Ther. 2019, 50, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Reist, C.; Gory, A.; Hollifield, M. Sleep-Disordered Breathing Impact on Efficacy of Prolonged Exposure Therapy for Posttraumatic Stress Disorder. J. Trauma. Stress 2017, 30, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Harb, G.C.; Cook, J.M.; Phelps, A.J.; Gehrman, P.R.; Forbes, D.; Localio, R.; Harpaz-Rotem, I.; Gur, R.C.; Ross, R.J. Randomized Controlled Trial of Imagery Rehearsal for Posttraumatic Nightmares in Combat Veterans. J. Clin. Sleep Med. 2019, 15, 757–767. [Google Scholar] [CrossRef]
- Yücel, D.E.; van Emmerik, A.A.; Souama, C.; Lancee, J. Comparative efficacy of imagery rehearsal therapy and prazosin in the treatment of trauma-related nightmares in adults: A meta-analysis of randomized controlled trials. Sleep Med. Rev. 2020, 50, 101248. [Google Scholar] [CrossRef] [PubMed]
- Krakow, B.; Melendrez, D.; Pedersen, B.; Johnston, L.; Hollifield, M.; Germain, A.; Koss, M.; Warner, T.D.; Schrader, R. Complex insomnia: Insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol. Psychiatry 2001, 49, 948–953. [Google Scholar] [CrossRef]
- Krakow, B.J.; Ulibarri, V.A.; Moore, B.A.; McIver, N.D. Posttraumatic stress disorder and sleep-disordered breathing: A review of comorbidity research. Sleep Med. Rev. 2015, 24, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jaoude, P.; Vermont, L.N.; Porhomayon, J.; El-Solh, A.A. Sleep-Disordered Breathing in Patients with Post-traumatic Stress Disorder. Ann. Am. Thorac. Soc. 2015, 12, 259–268. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Abu Salman, L.; Shulman, R.; Cohen, J.B. Obstructive Sleep Apnea, Hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and Management. Curr. Cardiol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Jehan, S.; Myers, A.K.; Zizi, F.; Pandi-Perumal, S.R.; Louis, G.J.; McFarlane, S.I. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: Epidemiology and pathophysiologic insights. Sleep Med. Disord. Int. J. 2018, 2, 52–58. [Google Scholar] [CrossRef]
- Gupta, M.A.; Simpson, F. Obstructive Sleep Apnea and Psychiatric Disorders: A Systematic Review. J. Clin. Sleep Med. 2015, 11, 165–175. [Google Scholar] [CrossRef]
- Young, T.; Blustein, J.; Finn, L.; Palta, M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997, 20, 608–613. [Google Scholar] [CrossRef] [Green Version]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.; Rapoport, D.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of Obstructive Sleep Apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, L.; Austin, D.; Peterson, A. Menopausal Status and Sleep-disordered Breathing in the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2003, 167, 1181–1185. [Google Scholar] [CrossRef]
- Zhang, Y.; Weed, J.G.; Ren, R.; Tang, X.; Zhang, W. Prevalence of obstructive sleep apnea in patients with posttraumatic stress disorder and its impact on adherence to continuous positive airway pressure therapy: A meta-analysis. Sleep Med. 2017, 36, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Krakow, B.; Melendrez, D.; Warner, T.D.; Clark, J.O.; Sisley, B.N.; Dorin, R.; Harper, R.M.; Leahigh, L.K.; Lee, S.A.; Sklar, D.; et al. Signs and Symptoms of Sleep-Disordered Breathing in Trauma Survivors: A matched comparison with classic sleep apnea patients. J. Nerv. Ment. Dis. 2006, 194, 433–439. [Google Scholar] [CrossRef]
- Lettieri, C.; Williams, S.G.; Collen, J.F. OSA Syndrome and Posttraumatic Stress Disorder: Clinical Outcomes and Impact of Positive Airway Pressure Therapy. Chest 2016, 149, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Series, F.; Roy, N.; Marc, I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am. J. Respir. Crit. Care Med. 1994, 150, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Trinder, J.; Whitworth, F.; Kay, A.; Wilkin, P. Respiratory instability during sleep onset. J. Appl. Physiol. 1992, 73, 2462–2469. [Google Scholar] [CrossRef]
- Thomson, S.; Morrell, M.J.; Cordingley, J.J.; Semple, S.J. Ventilation is unstable during drowsiness before sleep onset. J. Appl. Physiol. 2005, 99, 2036–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, D.J.; Younes, M.K. Arousal from sleep: Implications for obstructive sleep apnea pathogenesis and treatment. J. Appl. Physiol. 2014, 116, 302–313. [Google Scholar] [CrossRef]
- Foster, B.; Kravitz, S.; Collen, J.; Holley, A. 0490 Insomnia Among Military Members with OSA. Sleep 2019, 42, A196. [Google Scholar] [CrossRef]
- A El-Solh, A.; Lawson, Y.; Wilding, G.E. Impact of low arousal threshold on treatment of obstructive sleep apnea in patients with post-traumatic stress disorder. Sleep Breath. 2021, 25, 597–604. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003, 177, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Schredl, M.; Schmitt, J.; Hein, G.; Schmoll, T.; Eller, S.; Haaf, J. Nightmares and oxygen desaturations: Is sleep apnea related to heightened nightmare frequency? Sleep Breath. 2006, 10, 203–209. [Google Scholar] [CrossRef]
- Bahammam, A.S.; Almeneessier, A.S. Dreams and Nightmares in Patients with Obstructive Sleep Apnea: A Review. Front. Neurol. 2019, 10, 1127. [Google Scholar] [CrossRef]
- Fisher, S.; Lewis, K.; Bartle, I.; Ghosal, R.; Davies, L.; Blagrove, M. Emotional Content of Dreams in Obstructive Sleep Apnea Hypopnea Syndrome Patients and Sleepy Snorers attending a Sleep-Disordered Breathing Clinic. J. Clin. Sleep Med. 2011, 7, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BaHammam, A.S.; Al-Shimemeri, S.A.; Salama, R.I.; Sharif, M.M. Clinical and polysomnographic characteristics and response to continuous positive airway pressure therapy in obstructive sleep apnea patients with nightmares. Sleep Med. 2013, 14, 149–154. [Google Scholar] [CrossRef]
- Gross, M.; Lavie, P. Dreams in sleep apnea patients. Dreaming 1994, 4, 195–204. [Google Scholar] [CrossRef]
- Carrasco, E.; Santamaria, J.; Iranzo, A.; Pintor, L.; De Pablo, J.; Solanas, A.; Kumru, H.; Rodriguez, J.E.M.; Boget, T. Changes in dreaming induced by CPAP in severe obstructive sleep apnea syndrome patients. J. Sleep Res. 2006, 15, 430–436. [Google Scholar] [CrossRef]
- Walker, M.P.; Stickgold, R. Sleep-Dependent Learning and Memory Consolidation. Neuron 2004, 44, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Tempesta, D.; Socci, V.; De Gennaro, L.; Ferrara, M. Sleep and emotional processing. Sleep Med. Rev. 2018, 40, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.N.; Walker, M.P. The Role of Sleep in Emotional Brain Function. Annu. Rev. Clin. Psychol. 2014, 10, 679–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, U.; Gais, S.; Born, J. Emotional Memory Formation Is Enhanced across Sleep Intervals with High Amounts of Rapid Eye Movement Sleep. Learn. Mem. 2001, 8, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menz, M.; Rihm, J.; Salari, N.; Born, J.; Kalisch, R.; Pape, H.; Marshall, L.; Büchel, C. The role of sleep and sleep deprivation in consolidating fear memories. NeuroImage 2013, 75, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Murkar, A.L.; De Koninck, J. Consolidative mechanisms of emotional processing in REM sleep and PTSD. Sleep Med. Rev. 2018, 41, 173–184. [Google Scholar] [CrossRef]
- van der Helm, E.; Yao, J.; Dutt, S.; Rao, V.; Saletin, J.; Walker, M.P. REM Sleep Depotentiates Amygdala Activity to Previous Emotional Experiences. Curr. Biol. 2011, 21, 2029–2032. [Google Scholar] [CrossRef] [Green Version]
- Menz, M.M.; Rihm, J.S.; Büchel, C. REM Sleep Is Causal to Successful Consolidation of Dangerous and Safety Stimuli and Reduces Return of Fear after Extinction. J. Neurosci. 2016, 36, 2148–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace-Schott, E.F.; Milad, M.R.; Orr, S.P.; Rauch, S.L.; Stickgold, R.; Pitman, R.K. Sleep Promotes Generalization of Extinction of Conditioned Fear. Sleep 2009, 32, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Pace-Schott, E.F.; Germain, A.; Milad, M.R. Sleep and REM sleep disturbance in the pathophysiology of PTSD: The role of extinction memory. Biol. Mood Anxiety Disord. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace-Schott, E.F.; Germain, A.; Milad, M.R. Effects of sleep on memory for conditioned fear and fear extinction. Psychol. Bull. 2015, 141, 835–857. [Google Scholar] [CrossRef] [PubMed]
- Spoormaker, V.; Sturm, A.; Andrade, K.; Schröter, M.; Goya-Maldonado, R.; Holsboer, F.; Wetter, T.; Sämann, P.; Czisch, M. The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. J. Psychiatr. Res. 2010, 44, 1121–1128. [Google Scholar] [CrossRef]
- Norrholm, S.D.; Jovanovic, T. Fear Processing, Psychophysiology, and PTSD. Harv. Rev. Psychiatry 2018, 26, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.M.; DiGangi, J.A.; Phan, K.L. Functional Neuroanatomy of Emotion and Its Regulation in PTSD. Harv. Rev. Psychiatry 2018, 26, 116–128. [Google Scholar] [CrossRef]
- Germain, A. Sleep Disturbances as the Hallmark of PTSD: Where Are We Now? Am. J. Psychiatry 2013, 170, 372–382. [Google Scholar] [CrossRef]
- Ross, R.J.; Ball, W.A.; Dinges, D.F.; Kribbs, N.B.; Morrison, A.R.; Silver, S.M.; Mulvaney, F.D. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol. Psychiatry 1994, 35, 195–202. [Google Scholar] [CrossRef]
- Reist, C.; Jovanovic, T.; Kantarovich, D.; Weingast, L.; Hollifield, M.; Novin, M.; Khalaghizadeh, S.; Jafari, B.; George, R.; Riser, M.; et al. An analysis of fear inhibition and fear extinction in a sample of veterans with obstructive sleep apnea (OSA): Implications for co-morbidity with post-traumatic stress disorder (PTSD). Behav. Brain Res. 2021, 404, 113172. [Google Scholar] [CrossRef]
- Acierno, R.; Gros, D.F.; Ruggiero, K.J.; Dha, M.A.H.-T.; Knapp, R.G.; Lejuez, C.; Muzzy, W.; Frueh, C.B.; Egede, L.E.; Tuerk, P.W. Behavioral activation and therapeutic exposure for posttraumatic stress disorder: A noninferiority trial of treatment delivered in person versus home-based telehealth. Depress. Anxiety 2016, 33, 415–423. [Google Scholar] [CrossRef]
- Rauch, S.A.M.; Defever, E.; Favorite, T.; Duroe, A.; Garrity, C.; Martis, B.; Liberzon, I. Prolonged exposure for PTSD in a Veterans Health Administration PTSD clinic. J. Trauma. Stress 2009, 22, 60–64. [Google Scholar] [CrossRef]
- Tran, K.; Moulton, K.; Santesso, N.; Rabb, D. Cognitive Processing Therapy for Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK362346/ (accessed on 27 November 2021).
- Ho, F.Y.-Y.; Chan, C.S.; Tang, K.N.-S. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: A meta-analysis of randomized controlled trials. Clin. Psychol. Rev. 2016, 43, 90–102. [Google Scholar] [CrossRef]
- Baddeley, J.L.; Gros, D.F. Cognitive Behavioral Therapy for Insomnia as a Preparatory Treatment for Exposure Therapy for Posttraumatic Stress Disorder. Am. J. Psychother. 2013, 67, 203–214. [Google Scholar] [CrossRef]
- Gutner, C.A.; Pedersen, E.R.; Drummond, S. Going direct to the consumer: Examining treatment preferences for veterans with insomnia, PTSD, and depression. Psychiatry Res. 2018, 263, 108–114. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollicina, I.; Maniaci, A.; Lechien, J.R.; Iannella, G.; Vicini, C.; Cammaroto, G.; Cannavicci, A.; Magliulo, G.; Pace, A.; Cocuzza, S.; et al. Neurocognitive Performance Improvement after Obstructive Sleep Apnea Treatment: State of the Art. Behav. Sci. 2021, 11, 180. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Youakim, J.M.; Doghramji, K.; Schutte, S.L. Posttraumatic Stress Disorder and Obstructive Sleep Apnea Syndrome. J. Psychosom. Res. 1998, 39, 168–171. [Google Scholar] [CrossRef]
- Krakow, B.; Lowry, C.; Germain, A.; Gaddy, L.; Hollifield, M.; Koss, M.; Tandberg, D.; Johnston, L.; Melendrez, D. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J. Psychosom. Res. 2000, 49, 291–298. [Google Scholar] [CrossRef]
- Tamanna, S.; Parker, J.D.; Lyons, J.; Ullah, M.I. The Effect of Continuous Positive Air Pressure (CPAP) on Nightmares in Patients with Posttraumatic Stress Disorder (PTSD) and Obstructive Sleep Apnea (OSA). J. Clin. Sleep Med. 2014, 10, 631–636. [Google Scholar] [CrossRef] [Green Version]
- El-Solh, A.A.; Vermont, L.; Homish, G.; Kufel, T. The effect of continuous positive airway pressure on post-traumatic stress disorder symptoms in veterans with post-traumatic stress disorder and obstructive sleep apnea: A prospective study. Sleep Med. 2017, 33, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.; Smales, C.; Alexander, T.H.; Stepnowsky, C.; Pillar, G.; Malhotra, A.; Sarmiento, K.F. Treatment of OSA with CPAP Is Associated with Improvement in PTSD Symptoms among Veterans. J. Clin. Sleep Med. 2017, 13, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.I.; Campbell, D.G.; Bhagat, R.; Lyons, J.A.; Tamanna, S. Improving PTSD Symptoms and Preventing Progression of Subclinical PTSD to an Overt Disorder by Treating Comorbid OSA with CPAP. J. Clin. Sleep Med. 2017, 13, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, T.E.; Grunstein, R.R. Adherence to Continuous Positive Airway Pressure Therapy: The Challenge to Effective Treatment. Proc. Am. Thorac. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef] [Green Version]
- El-Solh, A.A.; Ayyar, L.; Akinnusi, M.; Relia, S.; Akinnusi, O. Positive Airway Pressure Adherence in Veterans with Posttraumatic Stress Disorder. Sleep 2010, 33, 1495–1500. [Google Scholar] [CrossRef] [Green Version]
- El-Solh, A.A.; Homish, G.G.; DiTursi, G.; Lazarus, J.; Rao, N.; Adamo, D.; Kufel, T. A Randomized Crossover Trial Evaluating Continuous Positive Airway Pressure Versus Mandibular Advancement Device on Health Outcomes in Veterans With Posttraumatic Stress Disorder. J. Clin. Sleep Med. 2017, 13, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- El-Solh, A.A.; Adamo, D.; Kufel, T. Comorbid insomnia and sleep apnea in Veterans with post-traumatic stress disorder. Sleep Breath. 2018, 22, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sharples, L.D.; Clutterbuck-James, A.L.; Glover, M.; Bennett, M.S.; Chadwick, R.; Pittman, M.A.; Quinnell, T.G. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef] [Green Version]
- Attali, V.; Chaumereuil, C.; Arnulf, I.; Golmard, J.-L.; Tordjman, F.; Morin, L.; Goudot, P.; Similowski, T.; Collet, J.-M. Predictors of long-term effectiveness to mandibular repositioning device treatment in obstructive sleep apnea patients after 1000 days. Sleep Med. 2016, 27–28, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gomis, J.; Willaert, E.; Nogues, L.; Pascual, M.; Somoza, M.; Monasterio, C. Five Years of Sleep Apnea Treatment with a Mandibular Advancement Device: Side effects and technical complications. Angle Orthod. 2010, 80, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Pliska, B.T.; Nam, H.; Chen, H.; Lowe, A.A.; Almeida, F.R. Obstructive Sleep Apnea and Mandibular Advancement Splints: Occlusal Effects and Progression of Changes Associated with a Decade of Treatment. J. Clin. Sleep Med. 2014, 10, 1285–1291. [Google Scholar] [CrossRef] [Green Version]
- Vanderveken, O.M.; Van De Heyning, P.; Braem, M.J. Retention of mandibular advancement devices in the treatment of obstructive sleep apnea: An in vitro pilot study. Sleep Breath. 2013, 18, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Marklund, M.; Franklin, K.A. Treatment of elderly patients with snoring and obstructive sleep apnea using a mandibular advancement device. Sleep Breath. 2014, 19, 403–405. [Google Scholar] [CrossRef]
- Patil, R.D.; Sarber, K.M.; Epperson, M.V.; Tabangin, M.; Altaye, M.; Mesa, F.; Ishman, S.L. Hypoglossal Nerve Stimulation: Outcomes in Veterans with Obstructive Sleep Apnea and Common Comorbid Post-Traumatic Stress Disorder. Laryngoscope 2021, 131, S1–S11. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Carter, S.G.; Berger, M.S.; Carberry, J.; Bilston, L.E.; Butler, J.; Tong, B.; Martins, R.T.; Fisher, L.P.; McKenzie, D.K.; Grunstein, R.R.; et al. Zopiclone Increases the Arousal Threshold without Impairing Genioglossus Activity in Obstructive Sleep Apnea. Sleep 2016, 39, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.R.; Sheikh, K.L.; Costan-Toth, C.; Forsthoefel, D.; Bridges, E.; Andrada, T.F.; Holley, A.B. Eszopiclone and Zolpidem Do Not Affect the Prevalence of the Low Arousal Threshold Phenotype. J. Clin. Sleep Med. 2017, 13, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmickl, C.N.; Lettieri, C.J.; Orr, J.E.; Deyoung, P.; Edwards, B.A.; Owens, R.L.; Malhotra, A. The Arousal Threshold as a Drug Target to Improve Continuous Positive Airway Pressure Adherence: Secondary Analysis of a Randomized Trial. Am. J. Respir. Crit. Care Med. 2020, 202, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.C.; Van Tyle, K.M. New pharmacologic agents for insomnia and hypersomnia. Curr. Opin. Pulm. Med. 2020, 26, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; White, D.P.; Winkelman, J.W. Antidepressants and Periodic Leg Movements of Sleep. Biol. Psychiatry 2005, 58, 510–514. [Google Scholar] [CrossRef]
- Department of Veterans Affairs; Department of Defense. VA/DOD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. 2017. Available online: www.tricare.mil (accessed on 28 November 2021).
- American Psychological Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. 2017. Available online: https://www.apa.org/ptsd-guideline/ptsd.pdf (accessed on 28 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCall, C.A.; Watson, N.F. A Narrative Review of the Association between Post-Traumatic Stress Disorder and Obstructive Sleep Apnea. J. Clin. Med. 2022, 11, 415. https://doi.org/10.3390/jcm11020415
McCall CA, Watson NF. A Narrative Review of the Association between Post-Traumatic Stress Disorder and Obstructive Sleep Apnea. Journal of Clinical Medicine. 2022; 11(2):415. https://doi.org/10.3390/jcm11020415
Chicago/Turabian StyleMcCall, Catherine A., and Nathaniel F. Watson. 2022. "A Narrative Review of the Association between Post-Traumatic Stress Disorder and Obstructive Sleep Apnea" Journal of Clinical Medicine 11, no. 2: 415. https://doi.org/10.3390/jcm11020415
APA StyleMcCall, C. A., & Watson, N. F. (2022). A Narrative Review of the Association between Post-Traumatic Stress Disorder and Obstructive Sleep Apnea. Journal of Clinical Medicine, 11(2), 415. https://doi.org/10.3390/jcm11020415





