Choroidal Melanocytic Hamartoma
Abstract
:1. Introduction
2. Methods
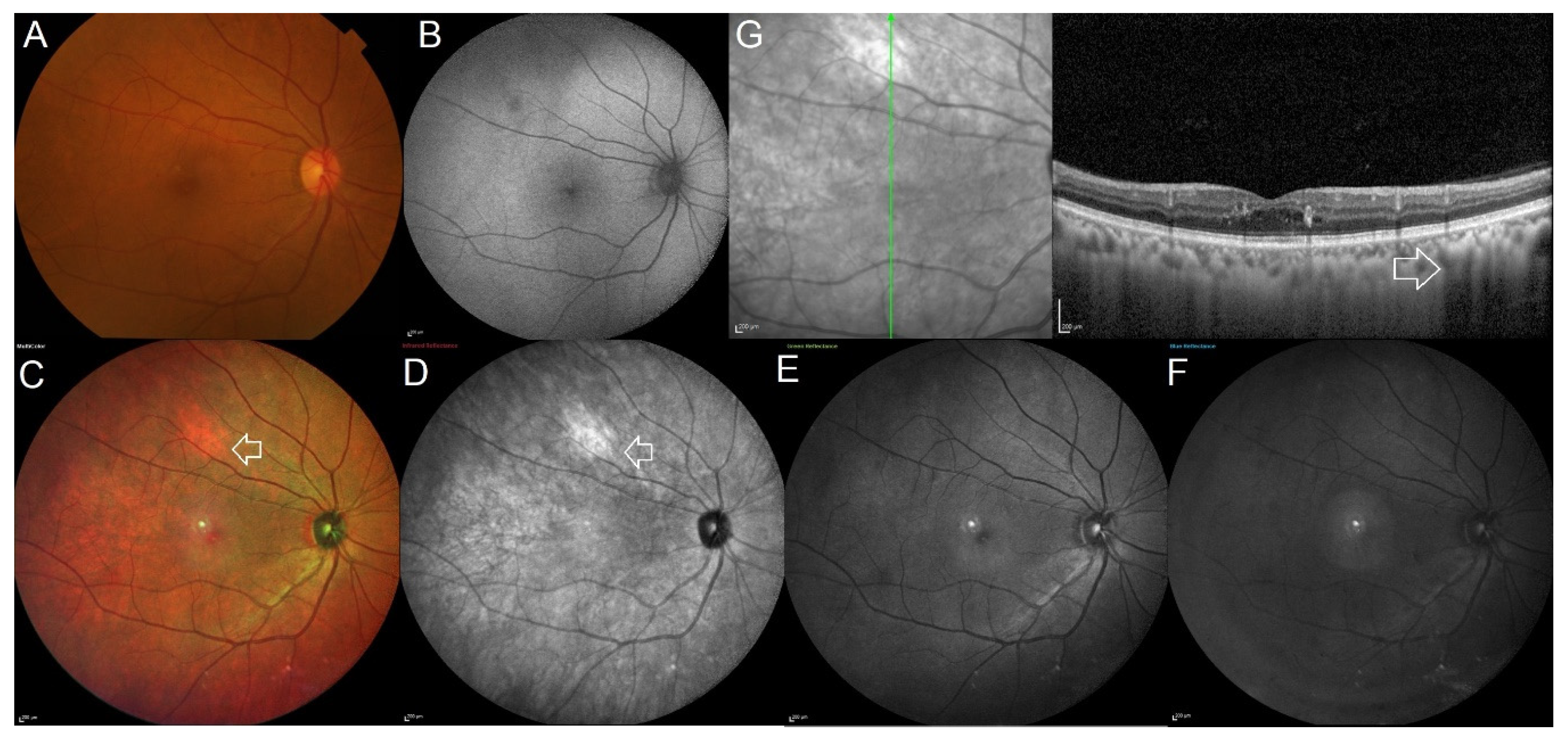
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Consent for Publication
Conflicts of Interest
References
- Weiter, J.J.; Delori, F.C.; Wing, G.L.; Fitch, K.A. Retinal Pigment Epithelial Lipofuscin and Melanin and Choroidal Melanin in Human Eyes. Investig. Ophthalmol. Vis. Sci. 1986, 27, 145–152. [Google Scholar]
- Traboulsi, E.I. Pigmented and Depigmented Lesions of the Ocular Fundus. Curr. Opin. Ophthalmol. 2012, 23, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, I.K.; Gaber, R.; Bartsch, D.-U.; Meshi, A.; Goldbaum, M.; Freeman, W.R. Comparison of Conventional Color Fundus Photography and Multicolor Imaging in Choroidal or Retinal Lesions. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, R.; Serranho, P.; Lobo, C. Digital Ocular Fundus Imaging: A Review. Ophthalmologica 2011, 226, 161–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.C.S.; Fleckenstein, M.; Schmitz-Valckenberg, S.; Holz, F.G. Clinical Application of Multicolor Imaging Technology. Ophthalmologica 2016, 236, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Moon, K.Y.; Jang, S.; Moon, Y. Comparison of MultiColor Fundus Imaging and Colour Fundus Photography in the Evaluation of Epiretinal Membrane. Acta Ophthalmol. 2019, 97, e533–e539. [Google Scholar] [CrossRef]
- Kilic Muftuoglu, I.; Bartsch, D.-U.; Barteselli, G.; Gaber, R.; Nezgoda, J.; Freeman, W.R. Visualization of macular pucker by multicolor scanning laser imaging. Retina 2018, 38, 352–358. [Google Scholar] [CrossRef]
- Venkatesh, R.; Sangai, S.; Pereira, A.; Jain, K.; Aseem, A.; Yadav, N.K. Differences in the Multicolour Imaging Features between the Superficial and Deep Vascular Occlusions. Int. Ophthalmol. 2020, 40, 3431–3439. [Google Scholar] [CrossRef]
- Saurabh, K.; Roy, R.; Goel, S. Correlation of Multicolor Images and Conventional Color Fundus Photographs with Foveal Autofluorescence Patterns in Diabetic Macular Edema. Indian J. Ophthalmol. 2020, 68, 141. [Google Scholar] [CrossRef]
- Pereira, A.; Thomas, S.; Yadav, N.; Venkatesh, R. Multicolor Imaging of Optic Disc Melanocytoma. Indian J. Ophthalmol. 2019, 67, 2056. [Google Scholar] [CrossRef]
- Thomas, N.; Ghosh, P.; Chowdhury, M.; Saurabh, K.; Roy, R. Multicolor Imaging in Optic Disc Swelling. Indian J. Ophthalmol. 2017, 65, 1251. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Wallman, J. The Multifunctional Choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.-N.; Simon, J.D.; Sarna, T. Role of Ocular Melanin in Ophthalmic Physiology and Pathology. Photochem. Photobiol. 2008, 84, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, L.; Ness, S.; Yi, J. Wavelength-Dependent Optical Properties of Melanosomes in Retinal Pigmented Epithelium and Their Changes with Melanin Bleaching: A Numerical Study. Biomed. Opt. Express 2017, 8, 3966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollias, N.; Baqer, A.H. Absorption Mechanisms of Human Melanin in the Visible, 400-720 Nm. J. Investig. Dermatol. 1987, 89, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, R.; Pereira, A.; Sangai, S.; Jain, K.; Gupta, I.; Aseem, A.; Singh, V.; Yadav, N.K. Variability in Imaging Findings in Choroidal Nevus Using Multicolor Imaging Vis-à-Vis Color Fundus Photography. J. Curr. Ophthalmol. 2020, 32, 285–289. [Google Scholar] [CrossRef]
- Venkatesh, R.; Pereira, A.; Sangai, S.; Singh, V.; Mahendradas, P.; Yadav, N.K. Enhanced Depth Imaging Optical Coherence Tomography and Multicolour Imaging Features in Bilateral Isolated Choroidal Melanocytosis. Clin. Exp. Optom. 2020, 104, 237–239. [Google Scholar] [CrossRef]
- Shields, C.L.; Manalac, J.; Das, C.; Ferguson, K.; Shields, J.A. Choroidal Melanoma: Clinical Features, Classification, and Top 10 Pseudomelanomas. Curr. Opin. Ophthalmol. 2014, 25, 177–185. [Google Scholar] [CrossRef]
- Venkatesh, R.; Reddy, N.G.; Chhablani, J. Impact of Melanin on Choroidal Measurements. Med. Hypotheses 2020, 146, 110408. [Google Scholar] [CrossRef]
- Jonna, G.; Daniels, A.B. Enhanced Depth Imaging OCT of Ultrasonographically Flat Choroidal Nevi Demonstrates 5 Distinct Patterns. Ophthalmol. Retina 2019, 3, 270–277. [Google Scholar] [CrossRef]
- DeSimone, J.D.; Dockery, P.W.; Kreinces, J.B.; Soares, R.R.; Shields, C.L. Survey of Ophthalmic Imaging Use to Assess Risk of Progression of Choroidal Nevus to Melanoma. Eye 2022. [CrossRef] [PubMed]
- Bindewald-Wittich, A.; Holz, F.G.; Ach, T.; Fiorentzis, M.; Bechrakis, N.E.; Willerding, G.D. Fundus Autofluorescence Imaging in Patients with Choroidal Melanoma. Cancers 2022, 14, 1809. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, J.J.; Trichopoulos, N.; Corrêa, Z.M.; Hershberger, V. Isolated Choroidal Melanocytosis: A Distinct Clinical Entity? Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Daitch, Z.; Shields, C.L.; Say, E.A.T.; Mashayekhi, A.; Shields, J.A. Submillimeter choroidal melanoma detection by enhanced depth imaging optical coherence tomography in a patient with oculodermal melanocytosis. Retin. Cases Brief Rep. 2016, 10, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; Vuong, V.S.; Oltjen, S.; Cunefare, D.; Farsiu, S.; Garzel, L.; Roberts, J.; Thomasy, S.M. Effect of Uveal Melanocytes on Choroidal Morphology in Rhesus Macaques and Humans on Enhanced-Depth Imaging Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5764–5771. [Google Scholar] [CrossRef] [Green Version]
- Abdolrahimzadeh, S.; Plateroti, A.M.; Recupero, S.M.; Lambiase, A. An Update on the Ophthalmologic Features in the Phakomatoses. J. Ophthalmol. 2016, 2016, 3043026. [Google Scholar] [CrossRef]


| Choroidal Nevus | Choroidal Melanoma | Isolated Choroidal Melanocytosis | Choroidal Melanocytic Hamartoma (Our Case Series) | |
|---|---|---|---|---|
| Age | Middle-age to elderly | Peaks at middle-age to elderly | Any age from infants to elderly | Young adults to elderly |
| Sex distribution | M = F | M > F | M < F | M > F |
| Symptoms | Asymptomatic | Usually asymptomatic but can present with vision loss, scotoma, photopsia or floaters. | Asymptomatic | Asymptomatic |
| Clinical features | Usually round, flat, small and grey colored lesion. 10% amelanotic. | Variable shape and size, often dark grey and irregular and raised lesion. 15% amelanotic. | Large, flat pigmented lesion with either focal or annular distribution with adjacent hypopigmented or depigmented lesion. | Poorly distinguished on clinical examination. |
| MultiColor® imaging | Salmon patch appearance. Sometimes can show variable presentation. | Lesion features like border, drusen and halo are similar to that seen on clinical examination. Size of the lesion appears lesser compared to clinical conventional fundus photograph. | Area of choroidal melanocytosis appears bright orange in color. | More obvious and distinct as a bright-orange colored lesion. |
| Optical coherence tomography features | Flat or dome shaped with diffuse hyperreflectivity, posterior shadowing, compressed choriocapillaris and rarely associated with drusen, subretinal fluid and/or choroidal neovascularization. | Smooth, dome-shaped destruction of the retinal pigment epithelium and outer retina, as well as associated subretinal fluid and “shaggy” photoreceptors. | There is increased hyperreflectivity in the choroid with poor visibility of underlying choroidal structures in the presence of choroidal melanocytosis. There is an obvious increase in choroidal thickness in the surrounding non-pigmented area, with poor visibility of choroidal architecture. There is no associated RPE elevation, drusen, orange pigment, or subretinal fluid. | Hyperreflectivity in the choroid with poor visibility of the choroidal structures beneath. The choroid around the lesion appears to be normal. There is no associated RPE elevation, drusen, orange pigment, or subretinal fluid. |
| Growth | Slow and minimal | Rapid | Slow and minimal | No growth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh, R.; Agrawal, S.; Reddy, N.G.; Mangla, R.; Yadav, N.K.; Chhablani, J. Choroidal Melanocytic Hamartoma. J. Clin. Med. 2022, 11, 5983. https://doi.org/10.3390/jcm11205983
Venkatesh R, Agrawal S, Reddy NG, Mangla R, Yadav NK, Chhablani J. Choroidal Melanocytic Hamartoma. Journal of Clinical Medicine. 2022; 11(20):5983. https://doi.org/10.3390/jcm11205983
Chicago/Turabian StyleVenkatesh, Ramesh, Sameeksha Agrawal, Nikitha Gurram Reddy, Rubble Mangla, Naresh Kumar Yadav, and Jay Chhablani. 2022. "Choroidal Melanocytic Hamartoma" Journal of Clinical Medicine 11, no. 20: 5983. https://doi.org/10.3390/jcm11205983






