Telemedicine and Digital Health Applications in Vascular Surgery
Abstract
:1. Introduction
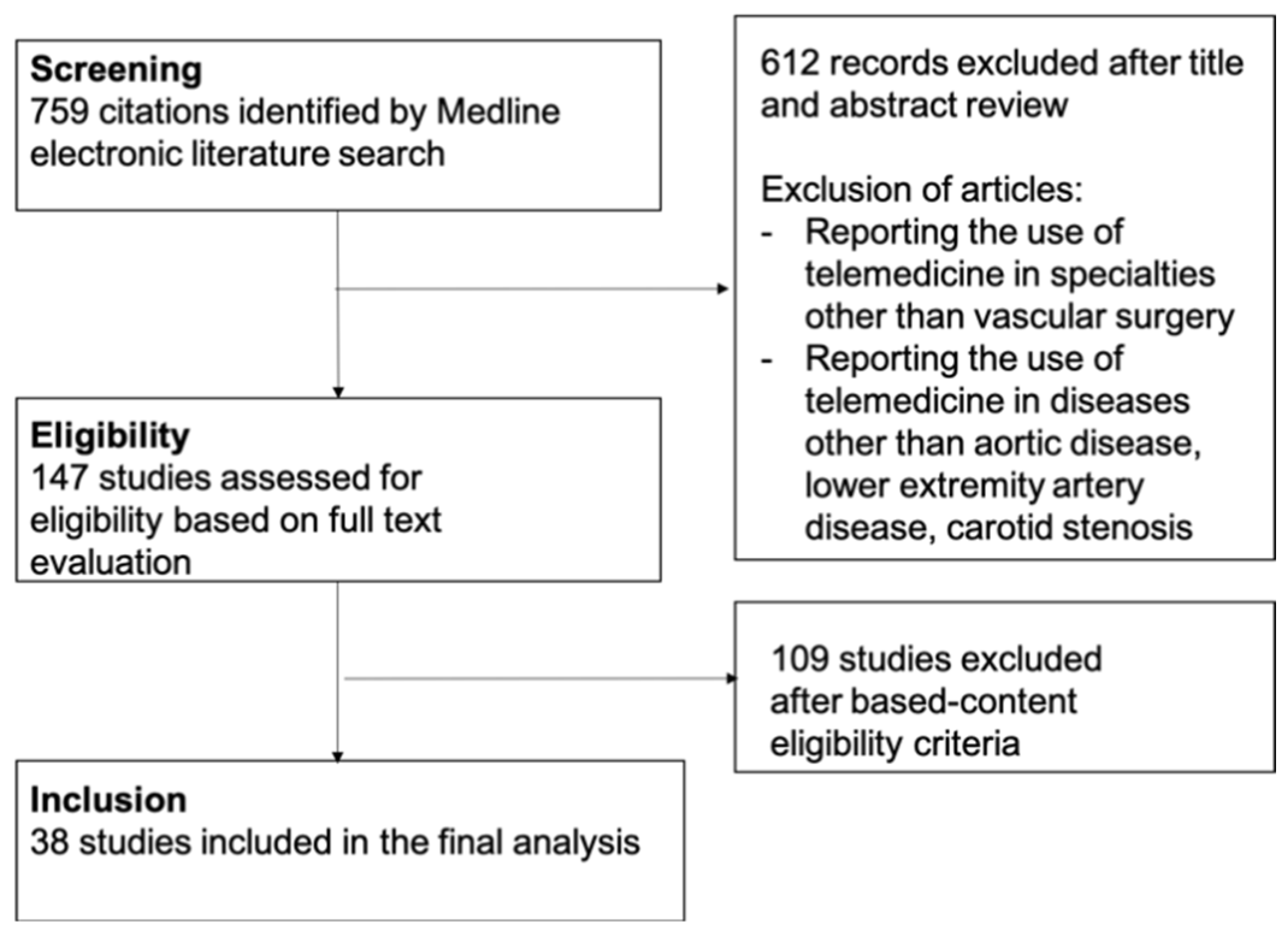
2. Methods
3. Telemedicine in Vascular Surgery
4. Telemedicine and Aortic Disease
4.1. Teleconsultation
4.2. Digital Tools for Information and Education of Patients with Aortic Disease
5. Telemedicine and Lower Extremity Artery Disease
5.1. Telemonitoring and Telecoaching to Enhance Exercise Program
5.2. Telemonitoring and Telecoaching to Enhance Follow-up
6. Telemedicine and Carotid Disease
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | attention control |
| LAEI | large artery elasticity index |
| NS | non-significant |
| PAD | peripheral artery disease |
| PASR | physical activity sedentary reduction |
| SET | supervised exercise therapy |
| SF-36 | Short-Form 36 Questionnaire |
| SUS | System Usability Scale |
| VascuQol | Vascular quality of life questionnaire |
| WIQ | Walking Impairment Questionnaire |
| WA | “go home and walk” advice group |
| WAM | wearable activity monitors |
References
- Telehealth is here to stay. Nat. Med. 2021, 27, 1121. [CrossRef] [PubMed]
- Dorsey, E.R.; Topol, E.J. State of Telehealth. N. Engl. J. Med. 2016, 375, 1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meij, E.; Anema, J.R.; Otten, R.H.; Huirne, J.A.; Schaafsma, F.G. The Effect of Perioperative E-Health Interventions on the Postoperative Course: A Systematic Review of Randomised and Non-Randomised Controlled Trials. PLoS ONE 2016, 11, e0158612. [Google Scholar]
- Marwaha, J.S.; Landman, A.B.; Brat, G.A.; Dunn, T.; Gordon, W.J. Deploying digital health tools within large, complex health systems: Key considerations for adoption and implementation. NPJ Digit. Med. 2022, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-T. Emerging New Era of Mobile Health Technologies. Healthc. Inform. Res. 2016, 22, 253–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maramba, I.D.; Jones, R.; Austin, D.; Edwards, K.; Meinert, E.; Chatterjee, A. The Role of Health Kiosks: Scoping Review. JMIR Med. Inform. 2022, 10, e26511. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [Green Version]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor's Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [Green Version]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [Green Version]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C., 3rd; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, R.J.; et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75, 4S–22S. [Google Scholar] [CrossRef]
- Ding, E.Y.; Pathiravasan, C.H.; Schramm, E.; Borrelli, B.; Liu, C.; Trinquart, L.; Kornej, J.; Benjamin, E.J.; Murabito, J.M.; McManus, D.D. Design, deployment, and usability of a mobile system for cardiovascular health monitoring within the electronic Framingham Heart Study. Cardiovasc. Digit. Health J. 2021, 2, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Kulnik, S.T.; Parvanov, E.D.; Fassl, A.; Eibensteiner, F.; Völkl-Kernstock, S.; Kletecka-Pulker, M.; Crutzen, R.; Gutenberg, J.; Höppchen, I.; et al. Research on Digital Technology Use in Cardiology: Bibliometric Analysis. J. Med. Internet Res. 2022, 24, e36086. [Google Scholar] [CrossRef]
- Gandapur, Y.; Kianoush, S.; Kelli, H.M.; Misra, S.; Urrea, B.; Blaha, M.J.; Graham, G.; Marvel, F.A.; Martin, S.S. The role of mHealth for improving medication adherence in patients with cardiovascular disease: A systematic review. Eur. Heart J. Qual. Care Clin. Outcomes 2016, 2, 237–244. [Google Scholar] [CrossRef]
- Palmer, M.J.; Machiyama, K.; Woodd, S.; Gubijev, A.; Barnard, S.; Russell, S.; Perel, P.; Free, C. Mobile phone-based interventions for improving adherence to medication prescribed for the primary prevention of cardiovascular disease in adults. Cochrane Database Syst. Rev. 2021, 3, CD012675. [Google Scholar] [CrossRef]
- Akinosun, A.S.; Polson, R.; Diaz-Skeete, Y.; De Kock, J.H.; Carragher, L.; Leslie, S.; Grindle, M.; Gorely, T. Digital Technology Interventions for Risk Factor Modification in Patients with Cardiovascular Disease: Systematic Review and Meta-analysis. JMIR mHealth uHealth 2021, 9, e21061. [Google Scholar] [CrossRef] [PubMed]
- Lareyre, F.; Behrendt, C.-A.; Chaudhuri, A.; Lee, R.; Carrier, M.; Adam, C.; Lê, C.D.; Raffort, J. Applications of artificial intelligence for patients with peripheral artery disease. J. Vasc. Surg. 2022, in press. [Google Scholar] [CrossRef]
- Lareyre, F.; Behrendt, C.-A.; Chaudhuri, A.; Ayache, N.; Delingette, H.; Raffort, J. Big Data and Artificial Intelligence in Vascular Surgery: Time for Multidisciplinary Cross-Border Collaboration. Angiology 2022, 73, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Feridooni, T.; Cuen-Ojeda, C.; Kishibe, T.; de Mestral, C.; Mamdani, M.; Al-Omran, M. Machine learning in vascular surgery: A systematic review and critical appraisal. NPJ Digit. Med. 2022, 5, 7. [Google Scholar] [CrossRef]
- Lareyre, F.; Lê, C.D.; Ballaith, A.; Adam, C.; Carrier, M.; Amrani, S.; Caradu, C.; Raffort, J. Applications of Artificial Intelligence in Non-cardiac Vascular Diseases: A Bibliographic Analysis. Angiology 2022, 73, 606–614. [Google Scholar] [CrossRef]
- Javidan, A.P.; Li, A.; Lee, M.H.; Forbes, T.L.; Naji, F. A Systematic Review and Bibliometric Analysis of Applications of Artificial Intelligence and Machine Learning in Vascular Surgery. Ann. Vasc. Surg. 2022, 85, 395–405. [Google Scholar] [CrossRef]
- Raffort, J.; Adam, C.; Carrier, M.; Ballaith, A.; Coscas, R.; Jean-Baptiste, E.; Hassen-Khodja, R.; Chakfé, N.; Lareyre, F. Artificial intelligence in abdominal aortic aneurysm. J. Vasc. Surg. 2020, 72, 321–333. [Google Scholar] [CrossRef]
- Tambyraja, A.L. Artificial intelligence in vascular surgery: The next gold rush or busted flush? J. Vasc. Surg. 2020, 72, 334. [Google Scholar] [CrossRef] [PubMed]
- Raffort, J.; Adam, C.; Carrier, M.; Lareyre, F. Fundamentals in Artificial Intelligence for Vascular Surgeons. Ann. Vasc. Surg. 2020, 65, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Association of American Medical Colleges. The Complexities of Physician Supply and Demand: Projections from 2018 to 2033; Association of American Medical Colleges: Washington, DC, USA, 2018. [Google Scholar]
- Adams, J.G.; Walls, R.M. Supporting the Health Care Workforce During the COVID-19 Global Epidemic. JAMA 2020, 323, 1439–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, C.L.; Sharma, V.; Sarfati, M.R.; Smith, B.K.; Kraiss, L.W.; McKellar, S.H.; Koliopoulou, A.; Brooke, B.S.; Selzman, C.H.; Glotzbach, J.P. Aortic disease in the time of COVID-19 and repercussions on patient care at an academic aortic center. J. Vasc. Surg. 2020, 72, 408–413. [Google Scholar] [CrossRef]
- Sterpetti, A.V. Telemedicine for screening and follow-up of abdominal aortic aneurysm. J. Vasc. Surg. 2022, 75, 1497. [Google Scholar] [CrossRef]
- Nishath, T.; Wright, K.; Burke, C.R.; Teng, X.; Cotter, N.; Yi, J.A.; Drudi, L.M.; Case, M.; David, C.C.; Fasano, M.; et al. Implementation of telemedicine in the care of patients with aortic dissection. Semin. Vasc. Surg. 2022, 35, 43–50. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Mendez Santos, I.; Monivas Palomero, V.; Calvo Iglesias, F. Telemedicine for patients with valvular heart disease or aortic disease in the era of COVID-19. Rev. Esp. Cardiol. 2021, 74, 361–362. [Google Scholar] [CrossRef]
- Hemingway, J.F.; Singh, N.; Starnes, B.W. Emerging practice patterns in vascular surgery during the COVID-19 pandemic. J. Vasc. Surg. 2020, 72, 396–402. [Google Scholar] [CrossRef]
- Fankhauser, G.T. Delivering high-quality vascular care by telehealth during the COVID-19 pandemic. J. Vasc. Surg. 2020, 72, 6–7. [Google Scholar] [CrossRef]
- Lin, J.C.; Welle, N.; Ding, J.; Chuen, J. A look to the future: Pandemic-induced digital technologies in vascular surgery. Semin. Vasc. Surg. 2021, 34, 139–151. [Google Scholar] [CrossRef]
- Castaneda, P.R.; Duffy, B.; Andraska, E.A.; Stevens, J.; Reschke, K.; Osborne, N.; Henke, P.K. Outcomes and safety of electronic consult use in vascular surgery. J. Vasc. Surg. 2020, 71, 1726–1732. [Google Scholar] [CrossRef]
- Chen, A.J.; Yeh, S.L.; Delfin, D.; Hoal, G.; Barron, N.; Riedinger, T.; Kashanijou, N.; Lieland, J.; Bickel, K.; O’Connell, J.B.; et al. Telemedicine and Vascular Surgery: Expanding Access and Providing Care Through the COVID-19 Pandemic. Am. Surg. 2022, 88, 2561–2564. [Google Scholar] [CrossRef] [PubMed]
- Endean, E.D.; Mallon, L.I.; Minion, D.J.; Kwolek, C.J.; Schwarcz, T.H. Telemedicine in vascular surgery: Does it work? Am. Surg. 2001, 67, 334–340. [Google Scholar]
- Lin, J.C.; Crutchfield, J.M.; Zurawski, D.K.; Stevens, C. Implementation of a virtual vascular clinic with point-of-care ultrasound in an integrated health care system. J. Vasc. Surg. 2018, 68, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gunter, R.L.; Fernandes-Taylor, S.; Rahman, S.; Awoyinka, L.; Bennett, K.M.; Weber, S.M.; Greenberg, C.C.; Kent, C.K. Feasibility of an Image-Based Mobile Health Protocol for Postoperative Wound Monitoring. J. Am. Coll. Surg. 2018, 226, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Gupta, B.; Ghosh, S.K. Chronic Wound Characterization using Bayesian Classifier under Telemedicine Framework. Int. J. e-Health Med. Commun. 2017, 7, 76–93. [Google Scholar] [CrossRef] [Green Version]
- Schnalzer, B.; Huber, S.; Sumerauer, I.; Preininger, M.; Alcalde, B.; Mischak, R. Evidence-Based Mobile Wound Application to Support Professionals in State-of-the-Art Chronic Wound Treatment. Stud. Health Technol. Inform. 2022, 293, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kostovich, C.T.; Etingen, B.; Wirth, M.; Patrianakos, J.; Kartje, R.; Baharestani, M.; Weaver, F.M. Outcomes of Telehealth for Wound Care: A Scoping Review. Adv. Ski. Wound Care 2022, 35, 394–403. [Google Scholar] [CrossRef]
- Gamus, A.; Keren, E.; Kaufman, H.; Brandin, G.; Peles, D.; Chodick, G. Telemedicine versus face-to-face care for treatment of patients with lower extremity ulcers. J. Wound Care 2021, 30, 916–921. [Google Scholar] [CrossRef]
- Stern, A.D.; Brönneke, J.; Debatin, J.F.; Hagen, J.; Matthies, H.; Patel, S.; Clay, I.; Eskofier, B.; Herr, A.; Hoeller, K.; et al. Advancing digital health applications: Priorities for innovation in real-world evidence generation. Lancet Digit. Health 2022, 4, e200–e206. [Google Scholar] [CrossRef]
- Gordon, N.P.; Hornbrook, M.C. Differences in Access to and Preferences for Using Patient Portals and Other eHealth Technologies Based on Race, Ethnicity, and Age: A Database and Survey Study of Seniors in a Large Health Plan. J. Med. Internet Res. 2016, 18, e50. [Google Scholar] [CrossRef]
- Murphy, D. Telemedicine and GDPR. Available online: https://challenge.ie/challengeblog/telemedicine-and-gdpr (accessed on 5 September 2022).
- Solimini, R.; Busardò, F.P.; Gibelli, F.; Sirignano, A.; Ricci, G. Ethical and Legal Challenges of Telemedicine in the Era of the COVID-19 Pandemic. Medicina 2021, 57, 1314. [Google Scholar] [CrossRef]
- Montemurro, N. Telemedicine: Could it represent a new problem for spine surgeons to solve? Glob. Spine J. 2022, 12, 1306–1307. [Google Scholar] [CrossRef]
- Gardiner, S.; Hartzell, T.L. Telemedicine and plastic surgery: A review of its applications, limitations and legal pitfalls. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, e47–e53. [Google Scholar] [CrossRef]
- Onor, M.L.; Misan, S. The Clinical Interview and the Doctor–Patient Relationship in Telemedicine. Telemed. J. e-Health 2005, 11, 102–105. [Google Scholar] [CrossRef]
- Kronenfeld, J.P.; Kang, N.; Kenel-Pierre, S.; Lopez, A.; Rey, J.; Fisher, F.; Karwowski, J.; Bornak, A. Establishing and maintaining a remote vascular surgery aortic program: A single-center 5-year experience at the Veterans Affairs. J. Vasc. Surg. 2022, 75, 1063–1072. [Google Scholar] [CrossRef]
- Chisci, E.; de Donato, G.; Fargion, A.; Ventoruzzo, G.; Parlani, G.; Setacci, C.; Ercolini, L.; Michelagnoli, S. One-year experience of a regional service model of teleconsultation for planning and treatment of complex thoracoabdominal aortic disease. J. Vasc. Surg. 2018, 67, 974–983. [Google Scholar] [CrossRef] [Green Version]
- Morley, J.; Floridi, L. Enabling digital health companionship is better than empowerment. Lancet Digit. Health 2019, 1, e155–e156. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, A.; Su, S.; Krauss, J. Utilizing Digital Health Technologies for Patient Education in Lifestyle Medicine. Am. J. Lifestyle Med. 2020, 14, 137–142. [Google Scholar] [CrossRef]
- Nilsson, O.; Hultgren, R.; Letterstal, A. eHealth tool for patients with abdominal aortic aneurysm: Development and initial evaluation. Scand. J. Caring Sci. 2020, 34, 348–356. [Google Scholar] [CrossRef]
- Nilsson, O.; Stenman, M.; Letterstål, A.; Hultgren, R. A randomized clinical trial of an eHealth intervention on anxiety in patients undergoing abdominal aortic aneurysm surgery. Br. J. Surg. 2021, 108, 917–924. [Google Scholar] [CrossRef]
- Mikkelsen, R.B.L.; Damsgaard, B.; Dahl, M. Patients’ perspectives show us how to care for their needs when living with an abdominal aortic aneurysm: Development of an eHealth solution. J. Vasc. Nurs. 2022, 40, 92–99. [Google Scholar] [CrossRef]
- Waddell, A.; Seed, S.; Broom, D.R.; McGregor, G.; Birkett, S.T.; Harwood, A.E. Safety of home-based exercise for people with intermittent claudication: A systematic review. Vasc. Med. 2021, 27, 186–192. [Google Scholar] [CrossRef]
- Haveman, M.E.; Kleiss, S.F.; Ma, K.F.; Vos, C.G.; Unlu, C.; Schuurmann, R.C.L.; Bokkers, R.P.H.; Hermens, H.J.; De Vries, J.-P.P.M. Telemedicine in patients with peripheral arterial disease: Is it worth the effort? Expert Rev. Med. Devices 2019, 16, 777–786. [Google Scholar] [CrossRef]
- Nugteren, M.J.; Catarinella, F.S.; Koning, O.H.J.; Hinnen, J.-W. Mobile applications in peripheral arterial disease (PAD): A review and introduction of a new innovative telemonitoring application: JBZetje. Expert Rev. Med. Devices 2021, 18, 581–586. [Google Scholar] [CrossRef]
- Aronow, W.S.; Avanesova, A.A.; Frishman, W.H.; Shamliyan, T.A. Inconsistent Benefits from Mobile Information Communication Technology in Adults with Peripheral Arterial Disease. Cardiol. Rev. 2022. [Google Scholar] [CrossRef]
- Kim, M.; Kim, C.; Kim, E.; Choi, M. Effectiveness of Mobile Health–Based Exercise Interventions for Patients with Peripheral Artery Disease: Systematic Review and Meta-Analysis. JMIR mHealth uHealth 2021, 9, e24080. [Google Scholar] [CrossRef]
- Chan, C.; Sounderajah, V.; Normahani, P.; Acharya, A.; Markar, S.R.; Darzi, A.; Bicknell, C.; Riga, C. Wearable Activity Monitors in Home Based Exercise Therapy for Patients with Intermittent Claudication: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 676–687. [Google Scholar] [CrossRef]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Scott, K.J.; Blevins, S.M. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: A randomized controlled trial. Circulation 2011, 123, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M. Step-Monitored Home Exercise Improves Ambulation, Vascular Function, and Inflammation in Symptomatic Patients with Peripheral Artery Disease: A Randomized Controlled Trial. J. Am. Heart Assoc. 2014, 3, e001107. [Google Scholar] [CrossRef] [Green Version]
- Nicolaï, S.P.; Teijink, J.A.; Prins, M.H.; Exercise Therapy in Peripheral Arterial Disease Study Group. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. J. Vasc. Surg. 2010, 52, 348–355. [Google Scholar] [CrossRef]
- Mays, R.J.; Hiatt, W.R.; Casserly, I.P.; Rogers, R.K.; Main, D.S.; Kohrt, W.M.; Ho, P.M.; Regensteiner, J.G. Community-based walking exercise for peripheral artery disease: An exploratory pilot study. Vasc. Med. 2015, 20, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Duscha, B.D.; Piner, L.W.; Patel, M.P.; Crawford, L.E.; Jones, W.S.; Patel, M.R.; Kraus, W.E. Effects of a 12-Week mHealth Program on Functional Capacity and Physical Activity in Patients with Peripheral Artery Disease. Am. J. Cardiol. 2018, 122, 879–884. [Google Scholar] [CrossRef]
- Normahani, P.; Kwasnicki, R.; Bicknell, C.; Allen, L.; Jenkins, M.P.; Gibbs, R.; Cheshire, N.; Darzi, A.; Riga, C. Wearable Sensor Technology Efficacy in Peripheral Vascular Disease (wSTEP): A Randomized Controlled Trial. Ann. Surg. 2018, 268, 1113–1118. [Google Scholar] [CrossRef]
- Laslovich, S.; Alvar, B.A.; Allison, M.; Rauh, M.J. Effects of Lifestyle Physical Activity on Vascular Function in Asymptomatic Peripheral Arterial Disease. Med. Sci. Sports Exerc. 2020, 52, 8–15. [Google Scholar] [CrossRef]
- Paldán, K.; Steinmetz, M.; Simanovski, J.; Rammos, C.; Ullrich, G.; Jánosi, R.A.; Moebus, S.; Rassaf, T.; Lortz, J. Supervised Exercise Therapy Using Mobile Health Technology in Patients with Peripheral Arterial Disease: Pilot Randomized Controlled Trial. JMIR mHealth uHealth 2021, 9, e24214. [Google Scholar] [CrossRef]
- Harzand, A.; Vakili, A.A.; Alrohaibani, A.; Abdelhamid, S.M.; Gordon, N.F.; Thiel, J.; Benarroch-Gampel, J.; Teodorescu, V.J.; Minton, K.; Wenger, N.K.; et al. Rationale and design of a smartphone-enabled, home-based exercise program in patients with symptomatic peripheral arterial disease: The smart step randomized trial. Clin. Cardiol. 2020, 43, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Paldán, K.; Simanovski, J.; Ullrich, G.; Steinmetz, M.; Rammos, C.; Jánosi, R.A.; Moebus, S.; Rassaf, T.; Lortz, J. Feasibility and Clinical Relevance of a Mobile Intervention Using TrackPAD to Support Supervised Exercise Therapy in Patients with Peripheral Arterial Disease: Study Protocol for a Randomized Controlled Pilot Trial. JMIR Res. Protoc. 2019, 8, e13651. [Google Scholar] [CrossRef] [Green Version]
- McDermott, M.M.; Spring, B.; Berger, J.S.; Treat-Jacobson, D.; Conte, M.S.; Creager, M.A.; Criqui, M.H.; Ferrucci, L.; Gornik, H.L.; Guralnik, J.M.; et al. Effect of a Home-Based Exercise Intervention of Wearable Technology and Telephone Coaching on Walking Performance in Peripheral Artery Disease: The HONOR Randomized Clinical Trial. JAMA 2018, 319, 1665–1676. [Google Scholar] [CrossRef]
- Tew, G.A.; Humphreys, L.; Crank, H.; Hewitt, C.; Nawaz, S.; Al-Jundi, W.; Trender, H.; Michaels, J.; Gorely, T. The development and pilot randomised controlled trial of a group education programme for promoting walking in people with intermittent claudication. Vasc. Med. 2015, 20, 348–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelis, N.; Buys, R.; Fourneau, I.; Dewit, T.; Cornelissen, V. Exploring physical activity behaviour—Needs for and interest in a technology-delivered, home-based exercise programme among patients with intermittent claudication. Vasa 2018, 47, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Alushi, K.; Hinterseher, I.; Peters, F.; Rother, U.; Bischoff, M.S.; Mylonas, S.; Grambow, E.; Gombert, A.; Busch, A.; Gray, D.; et al. Distribution of Mobile Health Applications amongst Patients with Symptomatic Peripheral Arterial Disease in Germany: A Cross-Sectional Survey Study. J. Clin. Med. 2022, 11, 498. [Google Scholar] [CrossRef]
- van den Houten, M.M.L.; Spruijt, S.; Fokkenrood, H.J.P.; Scheltinga, M.R.M.; Teijink, J.A.W. User Preferences for Mobile Health Interventions: A Survey among Intermittent Claudication Patients and Their Physical Therapists. Ann. Vasc. Surg. 2018, 46, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M. Telemedicine in the Cardiovascular World: Ready for the Future? Methodist Debakey Cardiovasc. J. 2020, 16, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F. The Benefits of Telemedicine in Personalized Prevention of Cardiovascular Diseases (CVD): A Systematic Review. J. Pers. Med. 2021, 11, 658. [Google Scholar] [CrossRef]
- Vernooij, J.W.; Kaasjager, H.A.; Van Der Graaf, Y.; Wierdsma, J.; Grandjean, H.M.; Hovens, M.M.; De Wit, G.A.; Visseren, F.L. Internet based vascular risk factor management for patients with clinically manifest vascular disease: Randomised controlled trial. BMJ 2012, 344, e3750. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-K.; Hung, C.-S.; Huang, C.-C.; Chen, Y.-H.; Wu, H.-W.; Yu, J.-Y.; Ho, Y.-L. The Costs and Cardiovascular Benefits in Patients with Peripheral Artery Disease from a Fourth-Generation Synchronous Telehealth Program: Retrospective Cohort Study. J. Med. Internet Res. 2021, 23, e24346. [Google Scholar] [CrossRef]
- Davins Riu, M.; Borras Perez, X.; Artigas Raventos, V.; Palomera Fanegas, E.; Serra Prat, M.; Alos Villacrosa, J. Use of Telehealth as a New Model for Following Intermittent Claudication and Promoting Patient Expertise. Telemed. J. e-Health 2018, 24, 773–781. [Google Scholar] [CrossRef]
- Greving, J.P.; Kaasjager, H.A.H.; Vernooij, J.W.P.; Hovens, M.M.C.; Wierdsma, J.; Grandjean, H.M.H.; Van Der Graaf, Y.; de Wit, G.A.; Visseren, F.L.J. Cost-effectiveness of a nurse-led internet-based vascular risk factor management programme: Economic evaluation alongside a randomised controlled clinical trial. BMJ Open 2015, 5, e007128. [Google Scholar] [CrossRef]
- Robaldo, A.; Rousas, N.; Pane, B.; Spinella, G.; Palombo, D. Telemedicine in vascular surgery: Clinical experience in a single centre. J. Telemed. Telecare 2010, 16, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor's Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef]
- Feil, K.; Rémi, J.; Küpper, C.; Herzberg, M.; Dorn, F.; Kunz, W.G.; Reidler, P.; Levin, J.; Hüttemann, K.; Tiedt, S.; et al. Inter-hospital transfer for mechanical thrombectomy within the supraregional stroke network NEVAS. J. Neurol. 2021, 268, 623–631. [Google Scholar] [CrossRef]
- Klingner, C.; Tinschert, P.; Brodoehl, S.; Berrouschot, J.; Witte, O.W.; Günther, A.; Klingner, C.M. The Effect of Endovascular Thrombectomy Studies on the Decision to Transfer Patients in a Telestroke Network. Telemed. e-Health 2020, 26, 388–394. [Google Scholar] [CrossRef]
- Troisi, N.; Cincotta, M.; Cardinali, C.; Battista, D.; Alberti, A.; Tramacere, L.; Michelagnoli, S.; Chisci, E. Reallocation of Carotid Surgery Activity with the Support of Telemedicine in a COVID-Free Clinic during COVID-19 Pandemic. Eur. Neurol. 2021, 84, 481–485. [Google Scholar] [CrossRef]
- English, S.W.; Barrett, K.M.; Freeman, W.D.; Demaerschalk, B.M. Telemedicine-enabled ambulances and mobile stroke units for prehospital stroke management. J. Telemed. Telecare 2022, 28, 458–463. [Google Scholar] [CrossRef]
- Antoniou, S.A.; Antoniou, G.A. Surgical Telementoring as a Means to Disseminate Vascular Expertise Around the World. J. Endovasc. Ther. 2017, 24, 859–860. [Google Scholar] [CrossRef]
- Lareyre, F.; Chaudhuri, A.; Adam, C.; Carrier, M.; Mialhe, C.; Raffort, J. Applications of Head-Mounted Displays and Smart Glasses in Vascular Surgery. Ann. Vasc. Surg. 2021, 75, 497–512. [Google Scholar] [CrossRef]
- Porretta, A.P.; Alerci, M.; Wyttenbach, R.; Antonucci, F.; Cattaneo, M.; Bogen, M.; Toderi, M.; Guerra, A.; Sartori, F.; Di Valentino, M.; et al. Long-term Outcomes of a Telementoring Program for Distant Teaching of Endovascular Aneurysm Repair. J. Endovasc. Ther. 2017, 24, 852–858. [Google Scholar] [CrossRef]
| Aim | Telemedicine Application | Methods | Main Outcomes Measured | Results | References |
|---|---|---|---|---|---|
| Compare changes in exercise performance and daily ambulatory activity in PAD patients with intermittent claudication after a home-based exercise program, a supervised exercise program and usual care control. |
|
|
|
| Gardner 2011 [62] |
| Compare changes in exercise performance in patients with symptomatic PAD following a home-based exercise program, a supervised exercise program and an attention control group. |
|
|
|
| Gardner 2014 [63] |
| Investigate the impact of provision of daily feedback with an accelerometer, in addition to supervised exercise therapy, on walking distance. |
|
|
|
| Nicolai 2010 [64] |
| Determine the efficacy of a community-based walking exercise program with training, monitoring and coaching components to improve exercise performance and patient-reported outcomes in PAD patients. |
|
|
|
| Mays 2015 [65] |
| Determine the effects on functional capacity and physical activity patterns of a 12-week m-Health program in PAD patients with intermittent claudication. |
|
|
|
| Duscha 2018 [66] |
| Evaluate the effect of using wearable activity monitors (WAMs) with supervised exercise programs in patients with intermittent claudication |
|
|
|
| Normahani 2018 [67] |
| Examine the effects of a 12-week in-home self-monitored physical activity in patients with asymptomatic PAD |
|
|
|
| Laslovich 2020 [68] |
| Evaluate changes in exercise performance using a smartphone app (TrackPAD) to support supervised exercise training in patients with PAD |
|
|
|
| Paldan 2021 [69] |
| Determine whether a home-based exercise intervention consisting of a wearable activity monitor and telephone coaching improves walking performances |
|
|
|
| McDermott 2018 [72] |
| Develop and pilot a group education program for promoting walking in people with intermittent claudication. |
|
|
|
| Tew 2015 [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lareyre, F.; Chaptoukaev, H.; Kiang, S.C.; Chaudhuri, A.; Behrendt, C.-A.; Zuluaga, M.A.; Raffort, J. Telemedicine and Digital Health Applications in Vascular Surgery. J. Clin. Med. 2022, 11, 6047. https://doi.org/10.3390/jcm11206047
Lareyre F, Chaptoukaev H, Kiang SC, Chaudhuri A, Behrendt C-A, Zuluaga MA, Raffort J. Telemedicine and Digital Health Applications in Vascular Surgery. Journal of Clinical Medicine. 2022; 11(20):6047. https://doi.org/10.3390/jcm11206047
Chicago/Turabian StyleLareyre, Fabien, Hava Chaptoukaev, Sharon C. Kiang, Arindam Chaudhuri, Christian-Alexander Behrendt, Maria A. Zuluaga, and Juliette Raffort. 2022. "Telemedicine and Digital Health Applications in Vascular Surgery" Journal of Clinical Medicine 11, no. 20: 6047. https://doi.org/10.3390/jcm11206047






