Persistent Atrial Fibrillation in Elderly Patients: Limited Efficacy of Pulmonary Vein Isolation
Abstract
1. Introduction
2. Methods
2.1. Study Design
Eligibility Criteria and Matching Criteria
2.2. Endpoints
2.3. Procedure
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Association of Clinical Parameters with Primary Efficacy Endpoint
3.3. Influence of Age on Primary Efficacy Endpoint
3.4. Primary Safety Endpoint
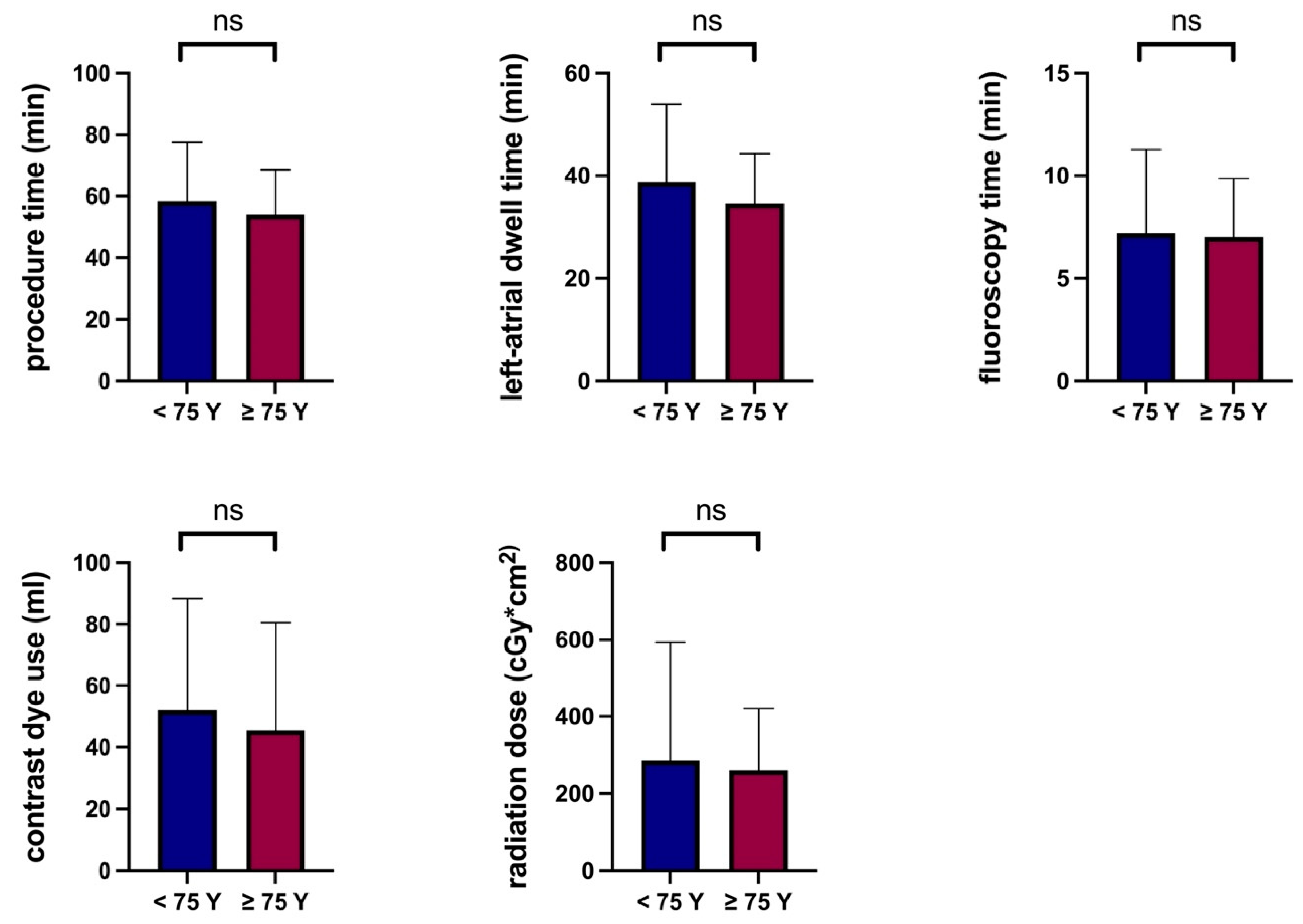
3.5. Procedural Parameters
4. Discussion
4.1. PVI in Elderly Patients
4.2. CryoPVI for Persistent AF
4.3. Pathophysiological Role of Age in Persistent AF
4.4. Alternative Therapeutic Strategies for Elderly Patients with Symptomatic Persistent AF
4.5. Safety
5. Strengths and limitations
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thrall, G.; Lane, D.; Carroll, D.; Lip, G.Y. Quality of life in patients with atrial fibrillation: A systematic review. Am. J. Med. 2006, 119, 448.e1–448.e19. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Verma, A.; Bhargava, M.; Saliba, W.; Bash, D.; Schweikert, R.; Brachmann, J.; Gunther, J.; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA 2005, 293, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Verma, A.; Connolly, S.J.; Kuck, K.H.; Nair, G.M.; Champagne, J.; Sterns, L.D.; Beresh, H.; Healey, J.S.; Natale, A.; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): A randomized trial. JAMA 2014, 311, 692–700. [Google Scholar] [CrossRef]
- Kuck, K.H.; Brugada, J.; Furnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Su, W.W.; Reddy, V.Y.; Bhasin, K.; Champagne, J.; Sangrigoli, R.M.; Braegelmann, K.M.; Kueffer, F.J.; Novak, P.; Gupta, S.K.; Yamane, T.; et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: Results from the multicenter STOP Persistent AF trial. Heart Rhythm 2020, 17, 1841–1847. [Google Scholar] [CrossRef]
- Omran, H.; Gutleben, K.J.; Molatta, S.; Fischbach, T.; Wellmann, B.; Horstkotte, D.; Korber, B.; Nolker, G. Second generation cryoballoon ablation for persistent atrial fibrillation: An updated meta-analysis. Clin. Res. Cardiol. 2018, 107, 182–192. [Google Scholar] [CrossRef]
- Tscholl, V.; Lin, T.; Lsharaf, A.K.; Bellmann, B.; Nagel, P.; Lenz, K.; Landmesser, U.; Roser, M.; Rillig, A. Cryoballoon ablation in the elderly: One year outcome and safety of the second-generation 28mm cryoballoon in patients over 75 years old. Europace 2018, 20, 772–777. [Google Scholar] [CrossRef]
- Kanda, T.; Masuda, M.; Kurata, N.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Tsujimura, T.; Okuno, S.; et al. Efficacy and safety of the cryoballoon-based atrial fibrillation ablation in patients aged >/=80 years. J. Cardiovasc. Electrophysiol. 2019, 30, 2242–2247. [Google Scholar] [CrossRef]
- Boon, R.A.; Iekushi, K.; Lechner, S.; Seeger, T.; Fischer, A.; Heydt, S.; Kaluza, D.; Treguer, K.; Carmona, G.; Bonauer, A.; et al. MicroRNA-34a regulates cardiac ageing and function. Nature 2013, 495, 107–110. [Google Scholar] [CrossRef]
- Biliczki, P.; Boon, R.A.; Girmatsion, Z.; Bukowska, A.; Ordog, B.; Kaess, B.M.; Hohnloser, S.H.; Goette, A.; Varro, A.; Moritz, A.; et al. Age-related regulation and region-specific distribution of ion channel subunits promoting atrial fibrillation in human left and right atria. Europace 2019, 21, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Willy, K.; Wasmer, K.; Dechering, D.G.; Kobe, J.; Lange, P.S.; Bogeholz, N.; Ellermann, C.; Reinke, F.; Frommeyer, G.; Eckardt, L. Ablation of paroxysmal and persistent atrial fibrillation in the very elderly real-world data on safety and efficacy. Clin. Cardiol. 2020, 43, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Metzner, I.; Wissner, E.; Tilz, R.R.; Rillig, A.; Mathew, S.; Schmidt, B.; Chun, J.; Wohlmuth, P.; Deiss, S.; Lemes, C.; et al. Ablation of atrial fibrillation in patients >/=75 years: Long-term clinical outcome and safety. Europace 2016, 18, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, T.; Nitta, J.; Nitta, G.; Kato, S.; Iwasaki, T.; Murata, K.; Junji, M.; Hirao, T.; Kanoh, M.; Takamiya, T.; et al. Propensity-matched comparison of cryoballoon and radiofrequency ablation for atrial fibrillation in elderly patients. Heart Rhythm 2019, 16, 838–845. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Vogler, J.; Willems, S.; Sultan, A.; Schreiber, D.; Luker, J.; Servatius, H.; Schaffer, B.; Moser, J.; Hoffmann, B.A.; Steven, D. Pulmonary Vein Isolation Versus Defragmentation: The CHASE-AF Clinical Trial. J. Am. Coll. Cardiol. 2015, 66, 2743–2752. [Google Scholar] [CrossRef]
- Providencia, R.; Lambiase, P.D. Persistent Atrial Fibrillation: Time to Stop Comparing Apples With Oranges. J. Am. Coll. Cardiol. 2016, 67, 2699–2700. [Google Scholar] [CrossRef]
- Boveda, S.; Metzner, A.; Nguyen, D.Q.; Chun, K.R.J.; Goehl, K.; Noelker, G.; Deharo, J.C.; Andrikopoulos, G.; Dahme, T.; Lellouche, N.; et al. Single-Procedure Outcomes and Quality-of-Life Improvement 12 Months Post-Cryoballoon Ablation in Persistent Atrial Fibrillation: Results From the Multicenter CRYO4PERSISTENT AF Trial. JACC Clin. Electrophysiol. 2018, 4, 1440–1447. [Google Scholar] [CrossRef]
- Tondo, C.; Iacopino, S.; Pieragnoli, P.; Molon, G.; Verlato, R.; Curnis, A.; Landolina, M.; Allocca, G.; Arena, G.; Fassini, G.; et al. Pulmonary vein isolation cryoablation for patients with persistent and long-standing persistent atrial fibrillation: Clinical outcomes from the real-world multicenter observational project. Heart Rhythm 2018, 15, 363–368. [Google Scholar] [CrossRef]
- de Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef]
- Kojodjojo, P.; Kanagaratnam, P.; Markides, V.; Davies, D.W.; Peters, N. Age-related changes in human left and right atrial conduction. J. Cardiovasc. Electrophysiol. 2006, 17, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kistler, P.M.; Sanders, P.; Fynn, S.P.; Stevenson, I.H.; Spence, S.J.; Vohra, J.K.; Sparks, P.B.; Kalman, J.M. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J. Am. Coll. Cardiol. 2004, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Akoum, N.; Mahnkopf, C.; Kholmovski, E.G.; Brachmann, J.; Marrouche, N.F. Age and sex differences in atrial fibrosis among patients with atrial fibrillation. Europace 2018, 20, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Cochet, H.; Mouries, A.; Nivet, H.; Sacher, F.; Derval, N.; Denis, A.; Merle, M.; Relan, J.; Hocini, M.; Haissaguerre, M.; et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J. Cardiovasc. Electrophysiol. 2015, 26, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Dun, W.; Yagi, T.; Rosen, M.R.; Boyden, P.A. Calcium and potassium currents in cells from adult and aged canine right atria. Cardiovasc. Res. 2003, 58, 526–534. [Google Scholar] [CrossRef]
- Anyukhovsky, E.P.; Sosunov, E.A.; Chandra, P.; Rosen, T.S.; Boyden, P.A.; Danilo, P., Jr.; Rosen, M.R. Age-associated changes in electrophysiologic remodeling: A potential contributor to initiation of atrial fibrillation. Cardiovasc. Res. 2005, 66, 353–363. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Zedda, A.; Huo, Y.; Kronborg, M.; Ulbrich, S.; Mayer, J.; Pu, L.; Richter, U.; Gaspar, T.; Piorkowski, J.; Piorkowski, C. Left Atrial Isolation and Appendage Occlusion in Patients With Atrial Fibrillation at End-Stage Left Atrial Fibrotic Disease. Circ. Arrhythmia Electrophysiol. 2021, 14, e010011. [Google Scholar] [CrossRef]
- Chatterjee, N.A.; Upadhyay, G.A.; Ellenbogen, K.A.; McAlister, F.A.; Choudhry, N.K.; Singh, J.P. Atrioventricular nodal ablation in atrial fibrillation: A meta-analysis and systematic review. Circ. Arrhythmia Electrophysiol. 2012, 5, 68–76. [Google Scholar] [CrossRef]

| Characteristic | Age < 75 years (n = 174) | Age ≥ 75 years (n = 94) | p |
|---|---|---|---|
| Age (years) | 64.5 ± 8 | 79.6 ± 3 | <0.001 |
| Male sex | 118 (67.8%) | 44 (46.8%) | 0.001 |
| Body mass index ≥ 30 kg/m2 | 57 (32.8%) | 12 (12.8%) | <0.001 |
| Antiarrhythmic drugs | |||
| (during/after blanking period) | |||
| overall | 82 (47.1%)/17 (9.8%) | 43 (45.7%)–6 (6.4%) | 0.9/0.5 |
| Flecainide | 29 (16.7%)/8 (4.6%) | 13 (13.8%)–0 (0%) | 0.6/0.055 |
| Amiodarone | 53 (30.5%)/9 (5.2%) | 28 (29.8%)–6 (6.4%) | 0.99/0.78 |
| Dronedarone | 2 (1.2%)/0 (0%) | 0 (0%)–0 (0%) | 0.54/0.99 |
| CHA2DS2-VASc score | 2.3 ± 1.3 | 3.9 ± 1.1 | <0.001 |
| LA-Diameter (mm) | 43 ± 9 | 45 ± 11 | 0.24 |
| Coronary artery disease | 23 (13.2%) | 30 (31.9%) | <0.001 |
| Heart failure (LVEF ≤ 40%) | 36 (20.7%) | 22 (23.4%) | 0.64 |
| Hypertension | 116 (61.3%) | 73 (78.8%) | 0.068 |
| Hyperlipidemia | 25 (14.4%) | 13 (13.8%) | 0.99 |
| Diabetes | 21 (12.1%) | 12 (12.8%) | 0.85 |
| Impaired renal function | 23 (13.2%) | 24 (25.5%) | 0.018 |
| Previous stroke | 8 (4.6%) | 8 (8.5%) | 0.28 |
| Characteristic | Persistent AF | |||
|---|---|---|---|---|
| p | Exp (B) | 95% CI for Exp (B) | ||
| Age (years) | 0.006 | 1.04 | 1.01 | 1.07 |
| Male sex | 0.53 | 0.86 | 0.54 | 1.38 |
| BMI > 30 km/m2 | 0.73 | 1.11 | 0.61 | 2.01 |
| LA Diameter (mm) | 0.33 | 0.99 | 0.97 | 1.01 |
| Coronary artery disease | 0.55 | 0.82 | 0.43 | 1.55 |
| Heart failure (LVEF ≤ 40%) | 0.94 | 1.03 | 0.56 | 1.90 |
| Hypertension | 0.07 | 0.65 | 0.40 | 1.04 |
| Hyperlipidemia | 0.66 | 0.85 | 0.43 | 1.69 |
| Diabetes | 0.44 | 0.69 | 0.26 | 1.81 |
| Impaired renal function | 0.41 | 0.80 | 0.47 | 1.36 |
| Characteristic | Age < 75 years (n = 69) | Age ≥ 75 years (n = 69) | p |
|---|---|---|---|
| Age (years) | 65.7 ± 8.4 | 79.5 ± 3.1 | <0.001 |
| Primary efficacy endpoints at 24 months | 13 (18.8%) | 31 (44.9%) | <0.001 |
| Male sex | 37 (53.6%) | 37 (53.6%) | 0.99 |
| Body mass index ≥ 30 kg/m2 | 5 (7.3%) | 5 (7.3%) | 0.99 |
| Antiarrhythmic drugs | |||
| (during/after blanking period) | |||
| overall | 30 (43.5%)/4 (5.8%) | 27 (39.1%)–6 (8.7%) | 0.73/0.75 |
| Flecainide | 9 (13%)/2 (2.9%) | 9 (13%)–0 (0%) | 0.99/0.5 |
| Amiodarone | 21 (30.4%)/2 (2.9%) | 17 (24.7%)–6 (8.7%) | 0.57/0.27 |
| Dronedarone | 0 (0%)/0 (0%) | 1 (1.5%)–0 (0%) | 0.99/0.99 |
| CHA2DS2-VASc score | 2.7 ± 1.4 | 3.8 ± 1.1 | <0.001 |
| LA-Diameter (mm) | 44 ± 9 | 44 ± 9 | 0.86 |
| Coronary artery disease | 18 (26.1%) | 18 (26.1%) | 0.99 |
| Heart failure (LVEF ≤ 40%) | 14 (20.3%) | 14 (20.3%) | 0.99 |
| Hypertension | 53 (76.8%) | 53 (76.8%) | 0.99 |
| Hyperlipidemia | 8 (11.6%) | 10 (14.5%) | 0.80 |
| Diabetes | 6 (8.7%) | 6 (8.7%) | 0.99 |
| Impaired renal function | 15 (21.7%) | 15 (21.7%) | 0.99 |
| Previous stroke | 5 (7.3%) | 8 (11.6%) | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boehmer, A.A.; Rothe, M.; Zezyk, C.; Soether, C.M.; Dobre, B.C.; Kaess, B.M.; Ehrlich, J.R. Persistent Atrial Fibrillation in Elderly Patients: Limited Efficacy of Pulmonary Vein Isolation. J. Clin. Med. 2022, 11, 6070. https://doi.org/10.3390/jcm11206070
Boehmer AA, Rothe M, Zezyk C, Soether CM, Dobre BC, Kaess BM, Ehrlich JR. Persistent Atrial Fibrillation in Elderly Patients: Limited Efficacy of Pulmonary Vein Isolation. Journal of Clinical Medicine. 2022; 11(20):6070. https://doi.org/10.3390/jcm11206070
Chicago/Turabian StyleBoehmer, Andreas A., Moritz Rothe, Celine Zezyk, Christina M. Soether, Bianca C. Dobre, Bernhard M. Kaess, and Joachim R. Ehrlich. 2022. "Persistent Atrial Fibrillation in Elderly Patients: Limited Efficacy of Pulmonary Vein Isolation" Journal of Clinical Medicine 11, no. 20: 6070. https://doi.org/10.3390/jcm11206070
APA StyleBoehmer, A. A., Rothe, M., Zezyk, C., Soether, C. M., Dobre, B. C., Kaess, B. M., & Ehrlich, J. R. (2022). Persistent Atrial Fibrillation in Elderly Patients: Limited Efficacy of Pulmonary Vein Isolation. Journal of Clinical Medicine, 11(20), 6070. https://doi.org/10.3390/jcm11206070





