Accurate and Reliable Assessment of Heart Rate in Real-Life Clinical Settings Using an Imaging Photoplethysmography
Abstract
:1. Introduction
2. Materials and Methods
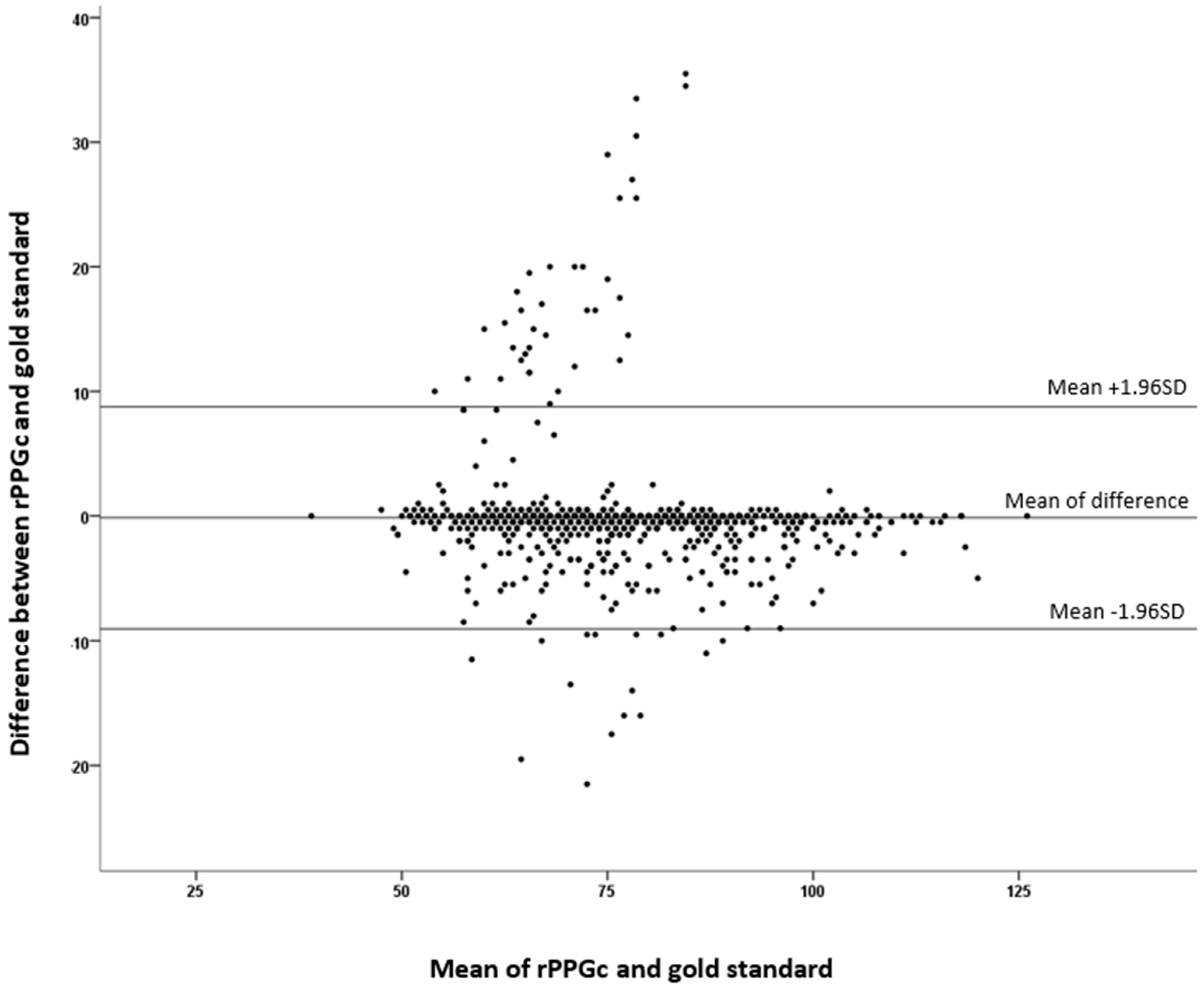
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jnr, B.A. Use of Telemedicine and Virtual Care for Remote Treatment in Response to COVID-19 Pandemic. J. Med. Syst. 2020, 44, 132. [Google Scholar] [CrossRef]
- Xu, S.; Rwei, A.Y.; Vwalika, B.; Chisembele, M.P.; Stringer, J.S.A.; Ginsburg, A.S.; Rogers, J.A. Wireless Skin Sensors for Physiological Monitoring of Infants in Low-Income and Middle-Income Countries. Lancet Digit. Health 2021, 3, e266–e273. [Google Scholar] [CrossRef]
- Garbey, M.; Sun, N.; Merla, A.; Pavlidis, I. Contact-Free Measurement of Cardiac Pulse Based on the Analysis of Thermal Imagery. IEEE Trans. Biomed. Eng. 2007, 54, 1418–1426. [Google Scholar] [CrossRef]
- Proceedings of the american physiological society. Am. J. Physiol.-Leg. Content 1937, 119, 257–427. [CrossRef] [Green Version]
- Humphreys, K.; Ward, T.; Markham, C. Noncontact Simultaneous Dual Wavelength Photoplethysmography: A Further Step toward Noncontact Pulse Oximetry. Rev. Sci. Instrum. 2007, 78, 044304. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.P.; Mastik, F.; van der Steen, A.F.W. Contactless Multiple Wavelength Photoplethysmographic Imaging: A First Step Toward “SpO2 Camera” Technology. Ann. Biomed. Eng. 2005, 33, 1034–1041. [Google Scholar] [CrossRef]
- Wu, T.; Blazek, V.; Schmitt, H.J. Photoplethysmography Imaging: A New Noninvasive and Noncontact Method for Mapping of the Dermal Perfusion Changes; Priezzhev, A.V., Oberg, P.A., Eds.; SPIE: Amsterdam, The Netherlands, 2000; p. 62. [Google Scholar]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote Plethysmographic Imaging Using Ambient Light. Opt. Express 2008, 16, 21434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas 2007, 28, R1–R39. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Papin, C.; Azorin-Peris, V.; Kalawsky, R.; Greenwald, S.; Hu, S. Use of Ambient Light in Remote Photoplethysmographic Systems: Comparison between a High-Performance Camera and a Low-Cost Webcam. J. Biomed. Opt. 2012, 17, 037005. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Yang, D.; Barszczyk, A.; Vempala, N.; Wei, J.; Wu, S.J.; Zheng, P.P.; Fu, G.; Lee, K.; Feng, Z.-P. Smartphone-Based Blood Pressure Measurement Using Transdermal Optical Imaging Technology. Circ. Cardiovasc. Imaging 2019, 12, e008857. [Google Scholar] [CrossRef]
- Coppetti, T.; Brauchlin, A.; Müggler, S.; Attinger-Toller, A.; Templin, C.; Schönrath, F.; Hellermann, J.; Lüscher, T.F.; Biaggi, P.; Wyss, C.A. Accuracy of Smartphone Apps for Heart Rate Measurement. Eur. J. Prev. Cardiolog. 2017, 24, 1287–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couderc, J.-P.; Kyal, S.; Mestha, L.K.; Xu, B.; Peterson, D.R.; Xia, X.; Hall, B. Detection of Atrial Fibrillation Using Contactless Facial Video Monitoring. Heart Rhythm. 2015, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.; Poorzargar, K.; Nagappa, M.; Saripella, A.; Parotto, M.; Englesakis, M.; Lee, K.; Chung, F. Effectiveness of Consumer-Grade Contactless Vital Signs Monitors: A Systematic Review and Meta-Analysis. J. Clin. Monit. Comput. 2022, 36, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Allado, E.; Poussel, M.; Moussu, A.; Saunier, V.; Bernard, Y.; Albuisson, E.; Chenuel, B. Innovative Measurement of Routine Physiological Variables (Heart Rate, Respiratory Rate and Oxygen Saturation) Using a Remote Photoplethysmography Imaging System: A Prospective Comparative Trial Protocol. BMJ Open 2021, 11, e047896. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Takano, C.; Ohta, Y. Heart Rate Measurement Based on a Time-Lapse Image. Med. Eng. Phys. 2007, 29, 853–857. [Google Scholar] [CrossRef]
- Da Costa, G. Optical Remote Sensing of Heartbeats. Opt. Commun. 1995, 117, 395–398. [Google Scholar] [CrossRef]
- Lim, Y.G.; Kim, K.K.; Park, S. ECG Measurement on a Chair without Conductive Contact. IEEE Trans. Biomed. Eng. 2006, 53, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, M. Monitoring of Electrocardiograms in Bed without Utilizing Body Surface Electrodes. IEEE Trans. Biomed. Eng. 1993, 40, 593–594. [Google Scholar] [CrossRef]
- Sjoding, M.W.; Dickson, R.P.; Iwashyna, T.J.; Gay, S.E.; Valley, T.S. Racial Bias in Pulse Oximetry Measurement. N. Engl. J. Med. 2020, 383, 2477–2478. [Google Scholar] [CrossRef] [PubMed]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating Sources of Inaccuracy in Wearable Optical Heart Rate Sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, A.; Prakash, S.K.A.; Jeni, L.A.; Tucker, C.S. Evaluation of Biases in Remote Photoplethysmography Methods. NPJ Digit. Med. 2021, 4, 91. [Google Scholar] [CrossRef] [PubMed]

| Female, n (%) | 471 (48.9%) |
|---|---|
| Age, mean (SD), years | 56.6 (±16.0) |
| Body mass index, mean (SD), kg/m2 | 28.1 (±7.3) |
| BMI < 30, n (%) | 650 (67.5%) |
| Class 1 obesity, n (%) | 172 (17.9%) |
| Class 2 obesity, n (%) | 67 (7.0%) |
| Class 3 obesity, n (%) | 74 (7.7%) |
| Fitzpatrick skin colour scale, n (%) | |
| 1 | 20 (2.1%) |
| 2 | 512 (3.2%) |
| 3 | 360 (37.4%) |
| 4 | 58 (6.0%) |
| 5 | 8 (0.8%) |
| 6 | 5 (0.5%) |
| n (%) | ICC | CI95 | |
|---|---|---|---|
| Ages groups, scale, n (%) | |||
| 18–29 years | 78 (8.1) | 0.883 | [0.817:0.925] |
| 30–39 years | 87 (9.0) | 0.804 | [0.700:0.872] |
| 40–49 years | 127 (13.2) | 0.942 | [0.917:0.959] |
| 50–59 years | 201 (20.9) | 0.875 | [0.836:0.906] |
| 60–69 years | 254 (26.4) | 0.842 | [0.798:0.876] |
| 70–79 years | 163 (16.9) | 0.912 | [0.880:0.935] |
| over 80 years | 53 (5.5) | 0.908 | [0.841:0.947] |
| ICC | CI95 | |
|---|---|---|
| Body mass index | ||
| BMI < 30 | 0.875 | [0.854:0.893] |
| Class 1 obesity | 0.955 | [0.940:0.967] |
| Class 2 obesity | 0.712 | [0.532:0.823] |
| Class 3 obesity | 0.979 | [0.966:0.987] |
| Fitzpatrick skin colour scale | ||
| 1 | 0.927 | [0.820:0.971] |
| 2 | 0.901 | [0.882:0.917] |
| 3 | 0.850 | [0.817:0.885] |
| 4 | 0.981 | [0.967:0.988] |
| 5 | 0.416 | [−1.644:0.881] |
| 6 | -0.204 | [−7.898:0.871] |
| Time | 30 s | 60 s | 120 s |
|---|---|---|---|
| 30 s | |||
| 60 s | 0.998 [0.998:0.998] | ||
| 120 s | 0.997 [0.996:0.997] | 0.999 [0.998:0.999] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allado, E.; Poussel, M.; Moussu, A.; Hily, O.; Temperelli, M.; Cherifi, A.; Saunier, V.; Bernard, Y.; Albuisson, E.; Chenuel, B. Accurate and Reliable Assessment of Heart Rate in Real-Life Clinical Settings Using an Imaging Photoplethysmography. J. Clin. Med. 2022, 11, 6101. https://doi.org/10.3390/jcm11206101
Allado E, Poussel M, Moussu A, Hily O, Temperelli M, Cherifi A, Saunier V, Bernard Y, Albuisson E, Chenuel B. Accurate and Reliable Assessment of Heart Rate in Real-Life Clinical Settings Using an Imaging Photoplethysmography. Journal of Clinical Medicine. 2022; 11(20):6101. https://doi.org/10.3390/jcm11206101
Chicago/Turabian StyleAllado, Edem, Mathias Poussel, Anthony Moussu, Oriane Hily, Margaux Temperelli, Asma Cherifi, Veronique Saunier, Yohann Bernard, Eliane Albuisson, and Bruno Chenuel. 2022. "Accurate and Reliable Assessment of Heart Rate in Real-Life Clinical Settings Using an Imaging Photoplethysmography" Journal of Clinical Medicine 11, no. 20: 6101. https://doi.org/10.3390/jcm11206101
APA StyleAllado, E., Poussel, M., Moussu, A., Hily, O., Temperelli, M., Cherifi, A., Saunier, V., Bernard, Y., Albuisson, E., & Chenuel, B. (2022). Accurate and Reliable Assessment of Heart Rate in Real-Life Clinical Settings Using an Imaging Photoplethysmography. Journal of Clinical Medicine, 11(20), 6101. https://doi.org/10.3390/jcm11206101






