8-Hydroxy-2-Deoxyguanosine and 8-Iso-Prostaglandin F2α: Putative Biomarkers to assess Oxidative Stress Damage Following Robot-Assisted Radical Prostatectomy (RARP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Population
2.3. Sample Preparation
2.4. 8-Hydroxy-2-Deoxyguanosine Measurement
2.5. 8-Iso-Prostaglandin f2α Measurement
2.6. Statistics
3. Results
3.1. Population
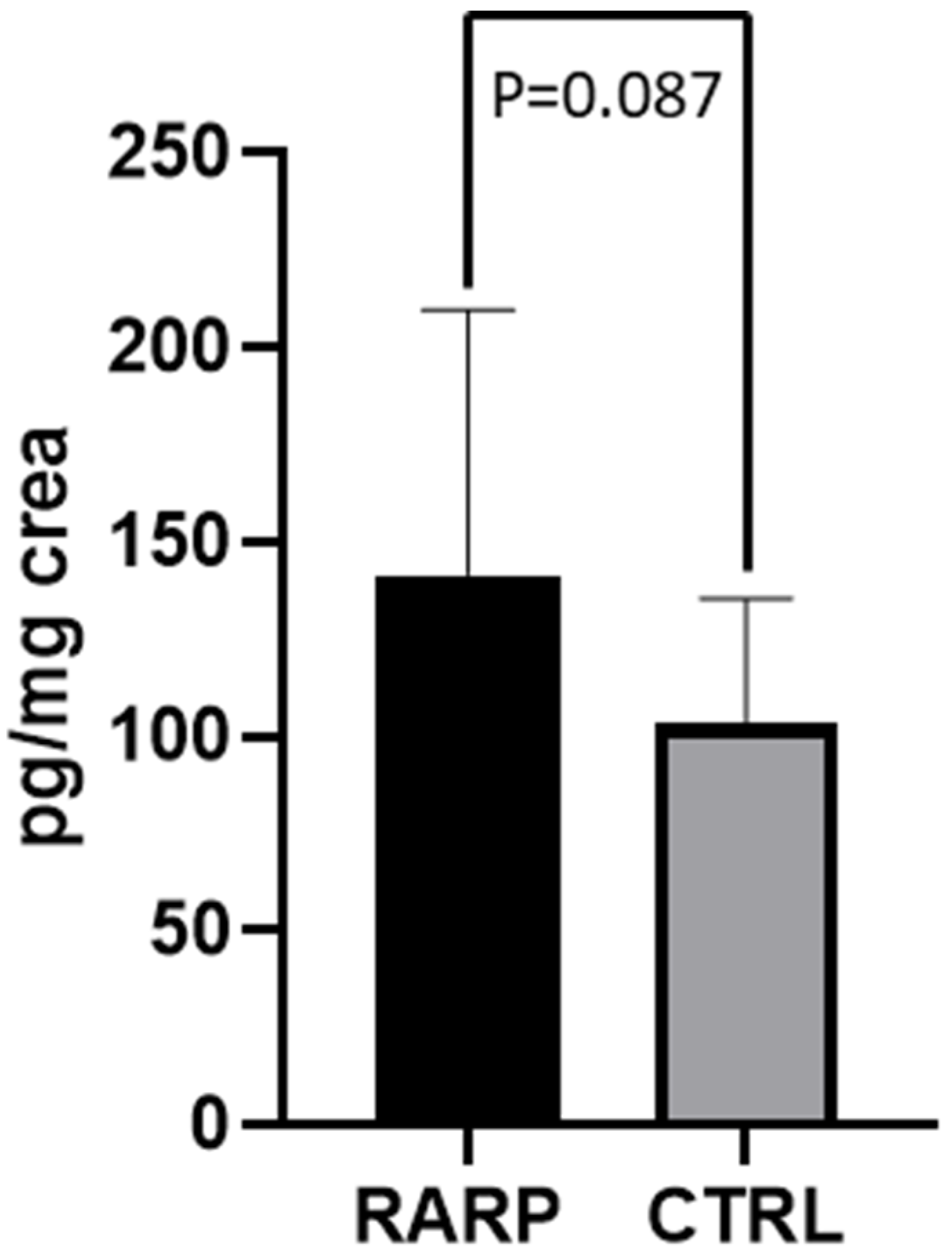
3.2. 8-OHdG Levels in Patients Undergoing RARP Surgery
3.3. 8-Iso-PGF2α Levels in Patients Undergoing RARP Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Ferro, M.; Crocetto, F.; Bruzzese, D.; Imbriaco, M.; Fusco, F.; Longo, N.; Napolitano, L.; La Civita, E.; Cennamo, M.; Liotti, A.; et al. Prostate Health Index and Multiparametric MRI: Partners in Crime Fighting Overdiagnosis and Overtreatment in Prostate Cancer. Cancers 2021, 13, 4723. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Miller, K.D.; Dale, W.; Mohile, S.G.; Cohen, H.J.; Leach, C.R.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J. Clin. 2019, 69, 452–467. [Google Scholar] [CrossRef] [Green Version]
- Tarantino, G.; Crocetto, F.; Vito, C.D.; Martino, R.; Pandolfo, S.D.; Creta, M.; Aveta, A.; Buonerba, C.; Imbimbo, C. Clinical factors affecting prostate-specific antigen levels in prostate cancer patients undergoing radical prostatectomy: A retrospective study. Future Sci. OA 2021, 7, FSO643. [Google Scholar] [CrossRef]
- Krishna, S.; Fan, Y.; Jarosek, S.; Adejoro, O.; Chamie, K.; Konety, B. Racial Disparities in Active Surveillance for Prostate Cancer. J. Urol. 2017, 197, 342–349. [Google Scholar] [CrossRef]
- Panigrahi, G.K.; Praharaj, P.P.; Kittaka, H.; Mridha, A.R.; Black, O.M.; Singh, R.; Mercer, R.; van Bokhoven, A.; Torkko, K.C.; Agarwal, C.; et al. Exosome proteomic analyses identify inflammatory phenotype and novel biomarkers in African American prostate cancer patients. Cancer Med. 2019, 8, 1110–1123. [Google Scholar] [CrossRef]
- Cao, L.; Yang, Z.; Qi, L.; Chen, M. Robot-assisted and laparoscopic vs open radical prostatectomy in clinically localized prostate cancer: Perioperative, functional, and oncological outcomes: A Systematic review and meta-analysis. Medicine 2019, 98, e15770. [Google Scholar] [CrossRef]
- Del Giudice, F.; Huang, J.; Li, S.; Sorensen, S.; Enemchukwu, E.; Maggi, M.; Salciccia, S.; Ferro, M.; Crocetto, F.; Pandolfo, S.D.; et al. Contemporary trends in the surgical management of urinary incontinence after radical prostatectomy in the United States. Prostate Cancer Prostatic Dis. 2022, 1–7. [Google Scholar] [CrossRef]
- Di Meo, N.A.; Loizzo, D.; Pandolfo, S.D.; Autorino, R.; Ferro, M.; Porta, C.; Stella, A.; Bizzoca, C.; Vincenti, L.; Crocetto, F.; et al. Metabolomic Approaches for Detection and Identification of Biomarkers and Altered Pathways in Bladder Cancer. Int. J. Mol. Sci. 2022, 23, 4173. [Google Scholar] [CrossRef]
- Di Minno, A.; Gelzo, M.; Caterino, M.; Costanzo, M.; Ruoppolo, M.; Castaldo, G. Challenges in Metabolomics-Based Tests, Biomarkers Revealed by Metabolomic Analysis, and the Promise of the Application of Metabolomics in Precision Medicine. Int. J. Mol. Sci. 2022, 23, 5213. [Google Scholar] [CrossRef]
- Loizzo, D.; Pandolfo, S.D.; Rogers, D.; Cerrato, C.; di Meo, N.A.; Autorino, R.; Mirone, V.; Ferro, M.; Porta, C.; Stella, A.; et al. Novel Insights into Autophagy and Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 3826. [Google Scholar] [CrossRef]
- Van Loon, B.; Markkanen, E.; Hubscher, U. Oxygen as a friend and enemy: How to combat the mutational potential of 8-oxo-guanine. DNA Repair 2010, 9, 604–616. [Google Scholar] [CrossRef]
- Oberley, T.D.; Zhong, W.; Szweda, L.I.; Oberley, L.W. Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate 2000, 44, 144–155. [Google Scholar] [CrossRef]
- Shukla, S.; Srivastava, J.K.; Shankar, E.; Kanwal, R.; Nawab, A.; Sharma, H.; Bhaskaran, N.; Ponsky, L.E.; Fu, P.; MacLennan, G.T.; et al. Oxidative Stress and Antioxidant Status in High-Risk Prostate Cancer Subjects. Diagnostics 2020, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Aydin, A.; Arsova-Sarafinovska, Z.; Sayal, A.; Eken, A.; Erdem, O.; Erten, K.; Ozgok, Y.; Dimovski, A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin. Biochem. 2006, 39, 176–179. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Oxidative Stress, Diet and Prostate Cancer. World J. Mens Health 2021, 39, 195–207. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Chen, W.; Jia, L.; Gupta, S.; MacLennan, G. The Role of Chronic Inflammation in Prostate Carcinogenesis: A Follow-Up Study. Ann. Urol. Oncol. 2019, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, S.; Kawahara, T.; Ishiguro, Y.; Takeshima, T.; Kuroda, S.; Izumi, K.; Miyamoto, H.; Uemura, H. Oxidative stress marker 8-hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Mol. Clin. Oncol. 2018, 9, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Kasai, H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res. 1997, 387, 147–163. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. Oxidative decay of DNA. J. Biol. Chem. 1997, 272, 19633–19636. [Google Scholar] [CrossRef] [Green Version]
- Camphausen, K.; Menard, C.; Sproull, M.; Goley, E.; Basu, S.; Coleman, C.N. Isoprostane levels in the urine of patients with prostate cancer receiving radiotherapy are not elevated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1536–1539. [Google Scholar] [CrossRef]
- Freedland, S.J.; Carducci, M.; Kroeger, N.; Partin, A.; Rao, J.Y.; Jin, Y.; Kerkoutian, S.; Wu, H.; Li, Y.; Creel, P.; et al. A double-blind, randomized, neoadjuvant study of the tissue effects of POMx pills in men with prostate cancer before radical prostatectomy. Cancer Prev. Res. 2013, 6, 1120–1127. [Google Scholar] [CrossRef] [Green Version]
- Di Minno, A.; Gentile, M.; Iannuzzo, G.; Calcaterra, I.; Tripaldella, M.; Porro, B.; Cavalca, V.; Di Taranto, M.D.; Tremoli, E.; Fortunato, G.; et al. Endothelial function improvement in patients with familial hypercholesterolemia receiving PCSK-9 inhibitors on top of maximally tolerated lipid lowering therapy. Thromb. Res. 2020, 194, 229–236. [Google Scholar] [CrossRef]
- Porro, B.; Di Minno, A.; Rocca, B.; Fiorelli, S.; Eligini, S.; Turnu, L.; Barbieri, S.; Parolari, A.; Tremoli, E.; Cavalca, V. Characterization of aspirin esterase activity in health and disease: In vitro and ex vivo studies. Biochem. Pharmacol. 2019, 163, 119–127. [Google Scholar] [CrossRef]
- Turnu, L.; Di Minno, A.; Porro, B.; Squellerio, I.; Bonomi, A.; Manega, C.M.; Werba, J.P.; Parolari, A.; Tremoli, E.; Cavalca, V. Assessing Free-Radical-Mediated DNA Damage during Cardiac Surgery: 8-Oxo-7,8-dihydro-2′-deoxyguanosine as a Putative Biomarker. Oxid. Med. Cell. Longev. 2017, 2017, 9715898. [Google Scholar] [CrossRef]
- Andreoli, R.; Manini, P.; De Palma, G.; Alinovi, R.; Goldoni, M.; Niessen, W.M.; Mutti, A. Quantitative determination of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine, 8-oxo-7,8-dihydroguanine, 8-oxo-7,8-dihydroguanosine, and their non-oxidized forms: Daily concentration profile in healthy volunteers. Biomarkers 2010, 15, 221–231. [Google Scholar] [CrossRef]
- Bogdanov, M.B.; Beal, M.F.; McCabe, D.R.; Griffin, R.M.; Matson, W.R. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2′-deoxyguanosine measurements in urine and other biologic matrices: A one-year evaluation of methods. Free Radic. Biol. Med. 1999, 27, 647–666. [Google Scholar] [CrossRef]
- Cavalca, V.; Minardi, F.; Scurati, S.; Guidugli, F.; Squellerio, I.; Veglia, F.; Dainese, L.; Guarino, A.; Tremoli, E.; Caruso, D. Simultaneous quantification of 8-iso-prostaglandin-F(2alpha) and 11-dehydro thromboxane B(2) in human urine by liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2010, 397, 168–174. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Shafik, N.M.; Elraheem, O.A.; Abou-Elnoeman, S.E. Association of paraoxonase-1(Q192R and L55M) gene polymorphisms and activity with colorectal cancer and effect of surgical intervention. Asian Pac. J. Cancer Prev. 2015, 16, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, H.; Tabur, S.; Ozkaya, M.; Aksoy, N.; Yildiz, H.; Akarsu, E. Paraoxonase and arylesterase activities in patients with papillary thyroid cancer. Scand. J. Clin. Lab. Investig. 2015, 75, 259–264. [Google Scholar] [CrossRef]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.D.; Lin, D.W.; Page, S.T.; Lundgren, A.D.; True, L.D.; Plymate, S.R. Oxidative DNA damage in the prostate may predispose men to a higher risk of prostate cancer. Transl. Oncol. 2009, 2, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Malins, D.C.; Johnson, P.M.; Barker, E.A.; Polissar, N.L.; Wheeler, T.M.; Anderson, K.M. Cancer-related changes in prostate DNA as men age and early identification of metastasis in primary prostate tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 5401–5406. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015, 289, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Kosova, F.; Temeltas, G.; Ari, Z.; Lekili, M. Possible relations between oxidative damage and apoptosis in benign prostate hyperplasia and prostate cancer patients. Tumour Biol. 2014, 35, 4295–4299. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.; Bagatini, M.D.; Reetz, L.G.; Chiesa, J.; Battisti, I.E.; Goncalves, J.F.; Duarte, M.M.; Schetinger, M.R.; Morsch, V.M. Oxidative stress and antioxidant status in prostate cancer patients: Relation to Gleason score, treatment and bone metastasis. Biomed. Pharmacother. 2011, 65, 516–524. [Google Scholar] [CrossRef]
- Bedir, F.; Kocaturk, H.; Altay, M.S.; Sebin, E.; Bedir, B. Serum paraoxonase 1 and 3 activities in benign and malignant diseases of the prostate and changes in levels following robotic-assisted laparoscopic radical prostatectomy. Turk. J. Med. Sci. 2020, 50, 1872–1878. [Google Scholar] [CrossRef]
- Costello, A.J. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat. Rev. Urol. 2020, 17, 177–188. [Google Scholar] [CrossRef]
- Ilic, D.; Evans, S.M.; Allan, C.A.; Jung, J.H.; Murphy, D.; Frydenberg, M. Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst. Rev. 2017, 9, CD009625. [Google Scholar] [CrossRef]
- McCormick, B.Z.; Chery, L.; Chapin, B.F. Contemporary outcomes following robotic prostatectomy for locally advanced and metastatic prostate cancer. Transl. Androl. Urol. 2021, 10, 2178–2187. [Google Scholar] [CrossRef]
- Ohta, T.; Kanda, H.; Sato, H.; Nishiwaki, M.; Okada, M.; Nakamura, K.; Yokota, Y.; Fukuzaki, H. Effect of valve replacement on left ventricular function in chronic aortic regurgitation. J. Cardiol. 1987, 17, 541–550. [Google Scholar]





| Overall Population Characteristics at Baseline | ||
|---|---|---|
| Variables | RARP (n = 40) | CTRL (n = 12) |
| Demographic characteristics | ||
| Age, years | 66.75 ± 6.61 | 69.25 ± 3.81 |
| BMI, kg/m2 | 27.14 ± 2.91 | 28.35 ± 2.56 |
| Diagnosis | ||
| Gleason Score | ||
| ≤6 no. (%) | 5 (12.5) | 0 (0) |
| 7 no. (%) | 3 (7.5) | 0 (0) |
| 8–10 no. (%) | 32 (80) | 0 (0) |
| t-score | ||
| 1 no. (%) | 0 (0) | 0 (0) |
| 2 no. (%) | 29 (72.5) | 0 (0) |
| 3 no. (%) | 11(27.5) | 0 (0) |
| PI-RADS | ||
| 1–2 no. (%) | 2 (5) | 0 (0) |
| 3 no. (%) | 2 (5) | 0 (0) |
| 4 no. (%) | 28(70) | 0 (0) |
| 5 no. (%) | 7 (17.5) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Minno, A.; Aveta, A.; Gelzo, M.; Tripodi, L.; Pandolfo, S.D.; Crocetto, F.; Imbimbo, C.; Castaldo, G. 8-Hydroxy-2-Deoxyguanosine and 8-Iso-Prostaglandin F2α: Putative Biomarkers to assess Oxidative Stress Damage Following Robot-Assisted Radical Prostatectomy (RARP). J. Clin. Med. 2022, 11, 6102. https://doi.org/10.3390/jcm11206102
Di Minno A, Aveta A, Gelzo M, Tripodi L, Pandolfo SD, Crocetto F, Imbimbo C, Castaldo G. 8-Hydroxy-2-Deoxyguanosine and 8-Iso-Prostaglandin F2α: Putative Biomarkers to assess Oxidative Stress Damage Following Robot-Assisted Radical Prostatectomy (RARP). Journal of Clinical Medicine. 2022; 11(20):6102. https://doi.org/10.3390/jcm11206102
Chicago/Turabian StyleDi Minno, Alessandro, Achille Aveta, Monica Gelzo, Lorella Tripodi, Savio Domenico Pandolfo, Felice Crocetto, Ciro Imbimbo, and Giuseppe Castaldo. 2022. "8-Hydroxy-2-Deoxyguanosine and 8-Iso-Prostaglandin F2α: Putative Biomarkers to assess Oxidative Stress Damage Following Robot-Assisted Radical Prostatectomy (RARP)" Journal of Clinical Medicine 11, no. 20: 6102. https://doi.org/10.3390/jcm11206102





