Management of Proximal Humerus Fractures in Adults—A Scoping Review
Abstract
1. Epidemiology
2. Classification
3. Clinical and Radiographic Assessment
4. Treatment Overview
5. Non-Operative Management
- There is contact or impaction between the head and shaft
- The humeral head is not dislocated
- There is minimal varus or valgus angulation of the humeral head (head shaft angle between 100 and 160 degrees)
- There is minimal displacement of any tuberosity fracture
- There is minimal articular surface involvement.
Complications
6. Operative Management
- Patient Factors
- 2.
- Fracture Severity
- 3.
- Surgeon Factors
6.1. Fractures with a Relative Indication for Operative Fixation
- Neer two-part surgical neck fractures with complete displacement of the humeral head relative to the shaft or incomplete displacement but no cortical continuity due to comminution [27]
- Displaced articular surface fracture with articular incongruity or more than 2 mm [26]
- Neer two-, three-, or four-part fracture with greater than 30 degrees varus or valgus angulation of the humeral head relative to the anatomic head shaft angle [26]
- Neer three- or four-part anterior fracture dislocation with retained soft tissue attachments to the humeral head [48]
- Neer three- or four-part posterior fracture dislocation with retained soft tissue attachments to the humeral head [44].
6.2. Fractures with a Relative Indication for Arthroplasty
7. Closed Reduction and Percutaneous Pinning
7.1. Indications
- (1)
- Two-part fractures
- (a)
- Greater Tuberosity
- (b)
- Lesser Tuberosity
- (c)
- Surgical Neck
- (2)
- Three-part surgical neck fractures with involvement of either the greater or lesser tuberosity
- (3)
- Valgus-impacted four-part fractures.
- (1)
- Good bone quality
- (2)
- Minimal comminution
- (3)
- Stable closed reduction
- (4)
- Intact medial calcar
- (5)
- Cooperative patient.
7.2. Contraindications
8. Surgical Approach
8.1. Patient Positioning
8.2. Fracture Reduction
8.2.1. Two-Part Greater and Lesser Tuberosity Fracture
8.2.2. Two-Part Surgical Neck Fractures
8.2.3. Three-Part Greater Tuberosity Fractures
8.2.4. Three-Part Lesser Tuberosity Fracture
8.2.5. Four-Part Valgus-Impacted Fracture
8.3. Fracture Fixation
8.4. Postoperative Management
8.5. Complications
8.6. Outcomes
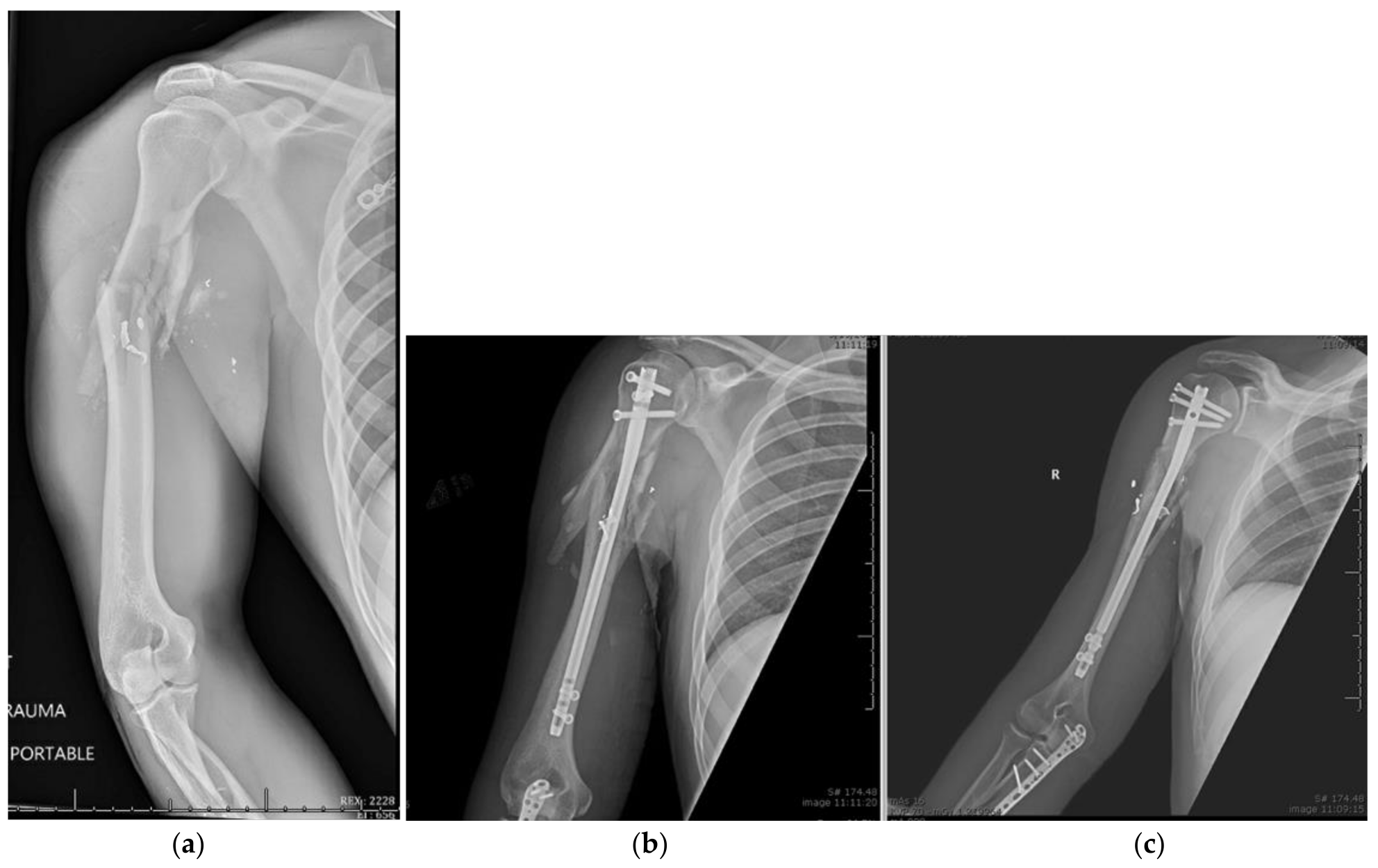
9. Open Reduction and Internal Fixation
10. Surgical Approach
10.1. Patient Positioning
10.2. Surgical Technique
10.3. Fracture Reduction
10.4. Fracture Fixation
10.5. Postoperative Management
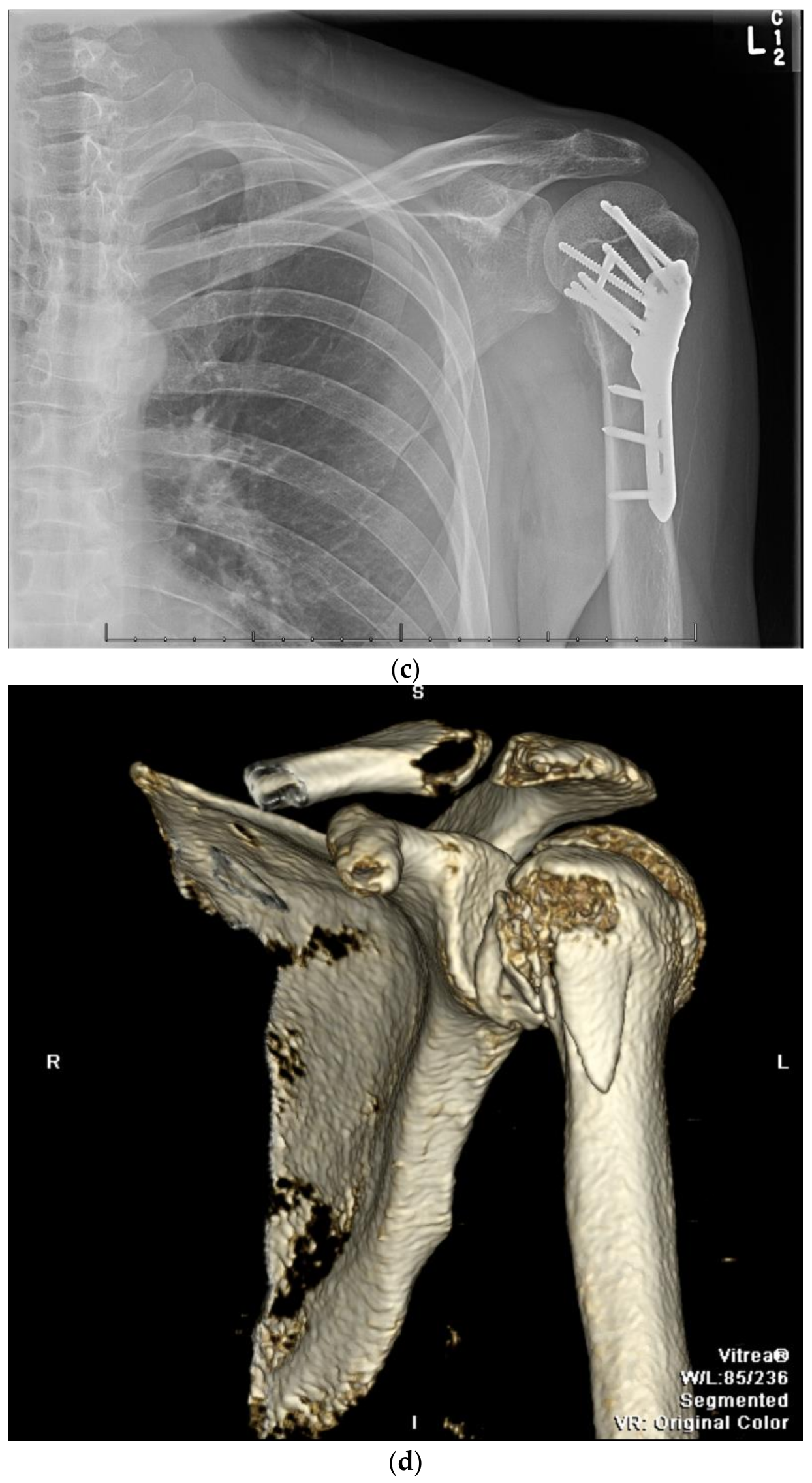
11. Open Reduction Internal Fixation with an Intramedullary Nail
12. Surgical Approach for ORIF with an Intramedullary Nail
12.1. Patient Positioning
12.2. Surgical Technique
12.3. Fracture Reduction
12.4. Fracture Fixation
12.5. Postoperative Management
12.6. Complications
12.7. Outcomes
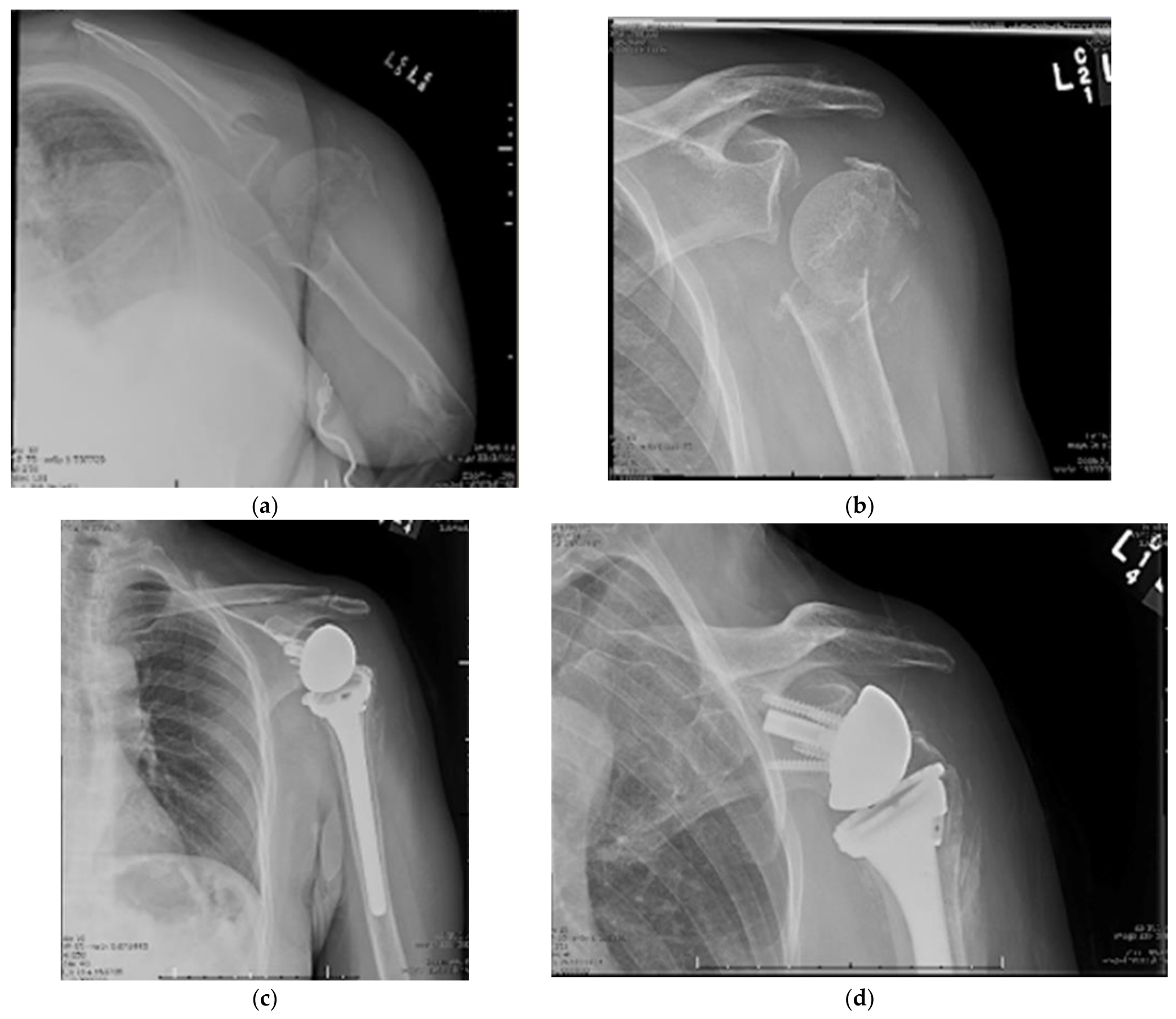
13. Reverse Total Shoulder Arthroplasty
14. Surgical Approach
14.1. Patient Positioning
14.2. Surgical Technique
14.3. Surgical Technique
14.4. Outcomes
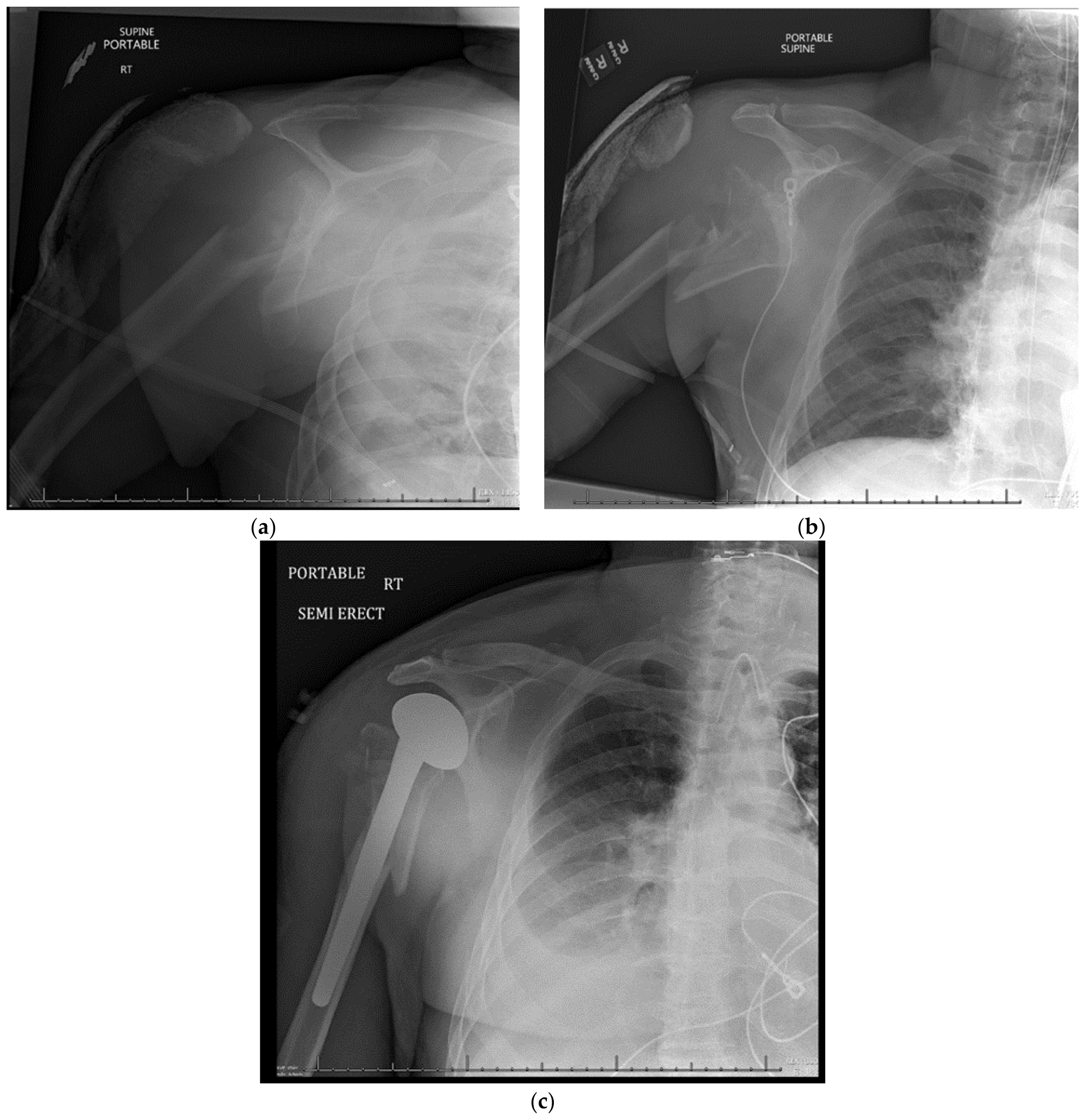
15. Hemiarthroplasty
15.1. Patient Positioning
15.2. Surgical Approach
15.3. Postoperative Management
15.4. Complications
15.5. Outcomes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Court-Brown, C.M.; Garg, A.; McQueen, M.M. The Epidemiology of Proximal Humeral Fractures. Acta Orthop. Scand. 2001, 72, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Passaretti, D.; Candela, V.; Sessa, P.; Gumina, S. Epidemiology of Proximal Humeral Fractures: A Detailed Survey of 711 Patients in a Metropolitan Area. J. Shoulder Elb. Surg. 2017, 26, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.H.; Wilder, J.H.; Ofa, S.A.; Lee, O.C.; Savoie, F.H.; O’Brien, M.J.; Sherman, W.F. Trending a Decade of Proximal Humerus Fracture Management in Older Adults. JSES Int. 2021, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Rodríguez, S.; Domínguez-Prado, D.M.; García-Reza, A.; Fernández-Fernández, D.; Pérez-Alfonso, E.; García-Piñeiro, J.; Castro-Menéndez, M. Epidemiology of Proximal Humerus Fractures. J. Orthop. Surg. Res. 2021, 16, 402. [Google Scholar] [CrossRef]
- McLean, A.S.; Price, N.; Graves, S.; Hatton, A.; Taylor, F.J. Nationwide Trends in Management of Proximal Humeral Fractures: An Analysis of 77,966 Cases from 2008 to 2017. J. Shoulder Elb. Surg. 2019, 28, 2072–2078. [Google Scholar] [CrossRef]
- Khatib, O.; Onyekwelu, I.; Zuckerman, J.D. The Incidence of Proximal Humeral Fractures in New York State from 1990 through 2010 with an Emphasis on Operative Management in Patients Aged 65 Years or Older. J. Shoulder Elb. Surg. 2014, 23, 1356–1362. [Google Scholar] [CrossRef]
- Codman, E.A. The Shoulder: Rupture of the Supraspinatus Tendon and Other Lesions in or about the Subacromial Bursa; T. Todd Company: Arlington, TX, USA, 1934. [Google Scholar]
- Neer, C. Displaced Proximal Humeral Fractures. J. Bone Jt. Surg. 1970, 52, 1077–1089. [Google Scholar] [CrossRef]
- Chung, S.W.; Han, S.S.; Lee, J.W.; Oh, K.-S.; Kim, N.R.; Yoon, J.P.; Kim, J.Y.; Moon, S.H.; Kwon, J.; Lee, H.-J.; et al. Automated Detection and Classification of the Proximal Humerus Fracture by Using Deep Learning Algorithm. Acta Orthop. 2018, 89, 468–473. [Google Scholar] [CrossRef]
- Gracitelli, M.E.C.; Dotta, T.A.G.; Assunção, J.H.; Malavolta, E.A.; Andrade-Silva, F.B.; Kojima, K.E.; Ferreira Neto, A.A. Intraobserver and Interobserver Agreement in the Classification and Treatment of Proximal Humeral Fractures. J. Shoulder Elb. Surg. 2017, 26, 1097–1102. [Google Scholar] [CrossRef]
- Muller, M.E. The Comprehensive Classification of Fractures of Long Bones; Springer: Berlin/Heidelberg, Germany, 1990; ISBN 978-3-540-18165-1. [Google Scholar]
- Hertel, R.; Hempfing, A.; Stiehler, M.; Leunig, M. Predictors of Humeral Head Ischemia after Intracapsular Fracture of the Proximal Humerus. J. Shoulder Elb. Surg. 2004, 13, 427–433. [Google Scholar] [CrossRef]
- Resch, H.; Beck, E.; Bayley, I. Reconstruction of the Valgus-Impacted Humeral Head Fracture. J. Shoulder Elb. Surg. 1995, 4, 73–80. [Google Scholar] [CrossRef]
- Resch, H.; Tauber, M.; Neviaser, R.J.; Neviaser, A.S.; Majed, A.; Halsey, T.; Hirzinger, C.; Al-Yassari, G.; Zyto, K.; Moroder, P. Classification of Proximal Humeral Fractures Based on a Pathomorphologic Analysis. J. Shoulder Elb. Surg. 2016, 25, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Majed, A.; Macleod, I.; Bull, A.M.J.; Zyto, K.; Resch, H.; Hertel, R.; Reilly, P.; Emery, R.J.H. Proximal Humeral Fracture Classification Systems Revisited. J. Shoulder Elb. Surg. 2011, 20, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.A.; Neviaser, R.J. Proximal Humerus Fractures: Evaluation and Management; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-319-08951-5. [Google Scholar]
- Maier, D.; Jaeger, M.; Izadpanah, K.; Strohm, P.C.; Suedkamp, N.P. Proximal Humeral Fracture Treatment in Adults. JBJS 2014, 96, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Schumaier, A.; Grawe, B. Proximal Humerus Fractures: Evaluation and Management in the Elderly Patient. Geriatr. Orthop. Surg. Rehabil. 2018, 9, 2151458517750516. [Google Scholar] [CrossRef]
- Visser, C.P.J.; Coene, L.N.J.E.M.; Brand, R.; Tavy, D.L.J. Nerve Lesions in Proximal Humeral Fractures. J. Shoulder Elb. Surg. 2001, 10, 421–427. [Google Scholar] [CrossRef]
- Neer, C.S. Four-Segment Classification of Proximal Humeral Fractures: Purpose and Reliable Use. J. Shoulder Elb. Surg. 2002, 11, 389–400. [Google Scholar] [CrossRef]
- Beks, R.B.; Ochen, Y.; Frima, H.; Smeeing, D.P.J.; van der Meijden, O.; Timmers, T.K.; van der Velde, D.; van Heijl, M.; Leenen, L.P.H.; Groenwold, R.H.H.; et al. Operative versus Nonoperative Treatment of Proximal Humeral Fractures: A Systematic Review, Meta-Analysis, and Comparison of Observational Studies and Randomized Controlled Trials. J. Shoulder Elb. Surg. 2018, 27, 1526–1534. [Google Scholar] [CrossRef]
- Jawa, A.; Burnikel, D. Treatment of Proximal Humeral Fractures: A Critical Analysis Review. JBJS Rev. 2016, 4, e2. [Google Scholar] [CrossRef]
- Handoll, H.H.G.; Ollivere, B.J.; Rollins, K.E. Interventions for Treating Proximal Humeral Fractures in Adults. Cochrane Database Syst. Rev. 2012, 12, CD000434. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; Cattermole, H.; McQueen, M.M. Impacted Valgus Fractures (B1.1) of the Proximal Humerus. The Results of Non-Operative Treatment. J. Bone Jt. Surg. Br. 2002, 84, 504–508. [Google Scholar] [CrossRef]
- Koval, K.J.; Gallagher, M.A.; Marsicano, J.G.; Cuomo, F.; McShinawy, A.; Zuckerman, J.D. Functional Outcome after Minimally Displaced Fractures of the Proximal Part of the Humerus. J. Bone Jt. Surg. Br. 1997, 79, 203–207. [Google Scholar] [CrossRef]
- Robinson, C.M.; Page, R.S. Severely Impacted Valgus Proximal Humeral Fractures: Results of Operative Treatment. JBJS 2003, 85, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Amin, A.K.; Godley, K.C.; Murray, I.R.; White, T.O. Modern Perspectives of Open Reduction and Plate Fixation of Proximal Humerus Fractures. J. Orthop. Trauma 2011, 25, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; McQueen, M.M.; Court-Brown, C.M. Minimally Displaced Proximal Humeral Fractures: Epidemiology and Outcome in 507 Cases. Acta Orthop. Scand. 2003, 74, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Tejwani, N.C.; Liporace, F.; Walsh, M.; France, M.A.; Zuckerman, J.D.; Egol, K.A. Functional Outcome Following One-Part Proximal Humeral Fractures: A Prospective Study. J. Shoulder Elb. Surg. 2008, 17, 216–219. [Google Scholar] [CrossRef]
- Platzer, P.; Kutscha-Lissberg, F.; Lehr, S.; Vecsei, V.; Gaebler, C. The Influence of Displacement on Shoulder Function in Patients with Minimally Displaced Fractures of the Greater Tuberosity. Injury 2005, 36, 1185–1189. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; McQueen, M.M. The Impacted Varus (A2.2) Proximal Humeral Fracture: Prediction of Outcome and Results of Nonoperative Treatment in 99 Patients. Acta Orthop. Scand. 2004, 75, 736–740. [Google Scholar] [CrossRef]
- Gardner, M.J.; Schmidt, A. Proximal Humerus Fractures and Shoulder Dislocations, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-1-60406-762-0. [Google Scholar]
- Sarmiento, A.; Zagorski, J.B.; Zych, G.A.; Latta, L.L.; Capps, C.A. Functional Bracing for the Treatment of Fractures of the Humeral Diaphysis. J. Bone Jt. Surg. Am. 2000, 82, 478–486. [Google Scholar] [CrossRef]
- Hanson, B.; Neidenbach, P.; de Boer, P.; Stengel, D. Functional Outcomes after Nonoperative Management of Fractures of the Proximal Humerus. J. Shoulder Elb. Surg. 2009, 18, 612–621. [Google Scholar] [CrossRef]
- Misra, A.; Kapur, R.; Maffulli, N. Complex Proximal Humeral Fractures in Adults—A Systematic Review of Management. Injury 2001, 32, 363–372. [Google Scholar] [CrossRef]
- Olerud, P.; Ahrengart, L.; Ponzer, S.; Saving, J.; Tidermark, J. Internal Fixation versus Nonoperative Treatment of Displaced 3-Part Proximal Humeral Fractures in Elderly Patients: A Randomized Controlled Trial. J. Shoulder Elb. Surg. 2011, 20, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Rangan, A.; Handoll, H.; Brealey, S.; Jefferson, L.; Keding, A.; Martin, B.C.; Goodchild, L.; Chuang, L.-H.; Hewitt, C.; Torgerson, D.; et al. Surgical vs Nonsurgical Treatment of Adults with Displaced Fractures of the Proximal Humerus: The PROFHER Randomized Clinical Trial. JAMA 2015, 313, 1037–1047. [Google Scholar] [CrossRef]
- Boileau, P.; Caligaris-Cordero, B.; Payeur, F.; Tinsi, L.; Argenson, C. Prognostic factors during rehabilitation after shoulder prostheses for fracture. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1999, 85, 106–116. [Google Scholar]
- Cummings, S.R.; Melton, L.J. Epidemiology and Outcomes of Osteoporotic Fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef]
- Volgas, D.A.; Stannard, J.P.; Alonso, J.E. Nonunions of the Humerus. Clin. Orthop. Relat. Res. 2004, 419, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Magovern, B.; Ramsey, M.L. Percutaneous Fixation of Proximal Humerus Fractures. Orthop. Clin. N. Am. 2008, 39, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Sidor, M.L.; Zuckerman, J.D.; Lyon, T.; Koval, K.; Cuomo, F.; Schoenberg, N. The Neer Classification System for Proximal Humeral Fractures. An Assessment of Interobserver Reliability and Intraobserver Reproducibility. J. Bone Jt. Surg. Am. 1993, 75, 1745–1750. [Google Scholar] [CrossRef]
- Jakob, R.P.; Miniaci, A.; Anson, P.S.; Jaberg, H.; Osterwalder, A.; Ganz, R. Four-Part Valgus Impacted Fractures of the Proximal Humerus. J. Bone Jt. Surg. Br. 1991, 73, 295–298. [Google Scholar] [CrossRef]
- Robinson, C.M.; Akhtar, A.; Mitchell, M.; Beavis, C. Complex Posterior Fracture-Dislocation of the Shoulder: Epidemiology, Injury Patterns, and Results of Operative Treatment. JBJS 2007, 89, 1454–1466. [Google Scholar] [CrossRef]
- Solberg, B.D.; Moon, C.N.; Franco, D.P.; Paiement, G.D. Locked Plating of 3- and 4-Part Proximal Humerus Fractures in Older Patients: The Effect of Initial Fracture Pattern on Outcome. J. Orthop. Trauma 2009, 23, 113–119. [Google Scholar] [CrossRef]
- Sporer, S.M.; Weinstein, J.N.; Koval, K.J. The Geographic Incidence and Treatment Variation of Common Fractures of Elderly Patients. JAAOS J. Am. Acad. Orthop. Surg. 2006, 14, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.A.; Patch, D.A.; Reed, L.A.; Spitler, C.A.; Horneff, J.G.; Ahn, J.; Strelzow, J.A.; Hebert-Davies, J.; Little, M.T.M.; Krause, P.C.; et al. Factors Influencing Surgical Management of Proximal Humerus Fractures: Do Shoulder and Trauma Surgeons Differ? J. Shoulder Elb. Surg. 2022, 31, e259–e269. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Khan, L.A.K.; Akhtar, M.A. Treatment of Anterior Fracture-Dislocations of the Proximal Humerus by Open Reduction and Internal Fixation. J. Bone Jt. Surg. Br. 2006, 88, 502–508. [Google Scholar] [CrossRef]
- Bastian, J.D.; Hertel, R. Initial Post-Fracture Humeral Head Ischemia Does Not Predict Development of Necrosis. J. Shoulder Elb. Surg. 2008, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.; de Miguel, I.; de la Cruz, J.J.; López-Martín, N. Percutaneous Fixation of Displaced Proximal Humeral Fractures: Indications Based on the Correlation between Clinical and Radiographic Results. J. Shoulder Elb. Surg. 2007, 16, 774–781. [Google Scholar] [CrossRef]
- Mellado, J.M.; Calmet, J.; García Forcada, I.L.; Saurí, A.; Giné, J. Early Intrathoracic Migration of Kirschner Wires Used for Percutaneous Osteosynthesis of a Two-Part Humeral Neck Fracture: A Case Report. Emerg. Radiol. 2004, 11, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Fenichel, I.; Oran, A.; Burstein, G.; Perry Pritsch, M. Percutaneous Pinning Using Threaded Pins as a Treatment Option for Unstable Two- and Three-Part Fractures of the Proximal Humerus: A Retrospective Study. Int. Orthop. SICO 2006, 30, 153–157. [Google Scholar] [CrossRef]
- Burkhead, W.Z.; Scheinberg, R.R.; Box, G. Surgical Anatomy of the Axillary Nerve. J. Shoulder Elb. Surg. 1992, 1, 31–36. [Google Scholar] [CrossRef]
- Robertson, D.D.; Yuan, J.; Bigliani, L.U.; Flatow, E.L.; Yamaguchi, K. Three-Dimensional Analysis of the Proximal Part of the Humerus: Relevance to Arthroplasty. JBJS 2000, 82, 1594. [Google Scholar] [CrossRef]
- Resch, H.; Povacz, P.; Fröhlich, R.; Wambacher, M. Percutaneous Fixation of Three- and Four-Part Fractures of the Proximal Humerus. J. Bone Jt. Surg. 1997, 79, 295–300. [Google Scholar] [CrossRef]
- Keener, J.D.; Parsons, B.O.; Flatow, E.L.; Rogers, K.; Williams, G.R.; Galatz, L.M. Outcomes after Percutaneous Reduction and Fixation of Proximal Humeral Fractures. J. Shoulder Elb. Surg. 2007, 16, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Rowles, D.J.; McGrory, J.E. Percutaneous Pinning of the Proximal Part of the Humerus: An Anatomic Study. JBJS 2001, 83, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Lyons, F.A.; Rockwood, C.A.J. Migration of Pins Used in Operations on the Shoulder. JBJS 1990, 72, 1262–1267. [Google Scholar] [CrossRef]
- Kralinger, F.; Irenberger, A.; Lechner, C.; Wambacher, M.; Golser, K.; Sperner, G. Vergleich der offenen vs. perkutanen Versorgung der Oberarmkopffraktur. Unfallchirurg 2006, 109, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Kamineni, S.; Ankem, H.; Sanghavi, S. Anatomical Considerations for Percutaneous Proximal Humeral Fracture Fixation. Injury 2004, 35, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Jaberg, H.; Warner, J.J.; Jakob, R.P. Percutaneous Stabilization of Unstable Fractures of the Humerus. J. Bone Jt. Surg. Am. 1992, 74, 508–515. [Google Scholar] [CrossRef]
- Hepp, P.; Theopold, J.; Voigt, C.; Engel, T.; Josten, C.; Lill, H. The Surgical Approach for Locking Plate Osteosynthesis of Displaced Proximal Humeral Fractures Influences the Functional Outcome. J. Shoulder Elb. Surg. 2008, 17, 21–28. [Google Scholar] [CrossRef]
- Robinson, C.M.; Khan, L.; Akhtar, A.; Whittaker, R. The Extended Deltoid-Splitting Approach to the Proximal Humerus. J. Orthop. Trauma 2007, 21, 657–662. [Google Scholar] [CrossRef]
- Gardner, M.J.; Boraiah, S.; Helfet, D.L.; Lorich, D.G. The Anterolateral Acromial Approach for Fractures of the Proximal Humerus. J. Orthop. Trauma 2008, 22, 132–137. [Google Scholar] [CrossRef]
- Gardner, M.J.; Boraiah, S.; Helfet, D.L.; Lorich, D.G. Indirect Medial Reduction and Strut Support of Proximal Humerus Fractures Using an Endosteal Implant. J. Orthop. Trauma 2008, 22, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Visconti, V.; Lombardi, L.V.; Ciccarelli, M.; Giudice, G. The Block-Bridge System: A New Concept and Surgical Technique to Reconstruct Articular Surfaces and Tuberosities in Complex Proximal Humeral Fractures. J. Shoulder Elb. Surg. 2008, 17, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, E.; Peraldi, P.; Naouri, J.F.; Rougereau, G.; Augereau, B. Four part valgus impacted fractures of the upper extremity of humerus: Ilium graft reconstruction. Apropos of 8 cases. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1996, 82, 658–662. [Google Scholar]
- Iannotti, J.P.; Ramsey, M.L.; Williams, G.R.; Warner, J.J. Nonprosthetic Management of Proximal Humeral Fractures. Instr. Course Lect. 2004, 53, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, F.; Gruber, G.; Schippinger, G.; Boldin, C.; Hofer, H.; Grechenig, W.; Szyszkowitz, R. Minimal-Invasive Treatment of Distal Femoral Fractures with the LISS (Less Invasive Stabilization System): A Prospective Study of 30 Fractures with a Follow up of 20 Months. Acta Orthop. Scand. 2004, 75, 56–60. [Google Scholar] [CrossRef]
- Gardner, M.J.; Weil, Y.; Barker, J.U.; Kelly, B.T.; Helfet, D.L.; Lorich, D.G. The Importance of Medial Support in Locked Plating of Proximal Humerus Fractures. J. Orthop. Trauma 2007, 21, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.; Nakatsuchi, Y.; Latta, L.; Milne, E. Distribution of Bone Mineral Density and Bone Strength of the Proximal Humerus. J. Shoulder Elb. Surg. 1994, 3, 234–242. [Google Scholar] [CrossRef]
- Tingart, M.J.; Lehtinen, J.; Zurakowski, D.; Warner, J.J.P.; Apreleva, M. Proximal Humeral Fractures: Regional Differences in Bone Mineral Density of the Humeral Head Affect the Fixation Strength of Cancellous Screws. J. Shoulder Elb. Surg. 2006, 15, 620–624. [Google Scholar] [CrossRef]
- Tingart, M.J.; Bouxsein, M.L.; Zurakowski, D.; Warner, J.P.; Apreleva, M. Three-Dimensional Distribution of Bone Density in the Proximal Humerus. Calcif. Tissue Int. 2003, 73, 531–536. [Google Scholar] [CrossRef]
- Sobel, A.D.; Shah, K.N.; Paxton, E.S. Fixation of a Proximal Humerus Fracture with an Intramedullary Nail. J. Orthop. Trauma 2017, 31, S47. [Google Scholar] [CrossRef]
- Gradl, G.; Dietze, A.; Kääb, M.; Hopfenmüller, W.; Mittlmeier, T. Is Locking Nailing of Humeral Head Fractures Superior to Locking Plate Fixation? Clin. Orthop. Relat. Res. 2009, 467, 2986–2993. [Google Scholar] [CrossRef] [PubMed]
- Rundquist, P.J.; Anderson, D.D.; Guanche, C.A.; Ludewig, P.M. Shoulder Kinematics in Subjects with Frozen Shoulder. Arch. Phys. Med. Rehabil. 2003, 84, 1473–1479. [Google Scholar] [CrossRef]
- Burkhart, S.S. Arthroscopic Subscapularis Tenolysis: A Technique for Treating Refractory Glenohumeral Stiffness Following Open Reduction and Internal Fixation of a Displaced Three-Part Proximal Humerus Fracture. Arthroscopy 1996, 12, 87–91. [Google Scholar] [CrossRef]
- Wijgman, A.J.; Roolker, W.; Patt, T.W.; Raaymakers, E.L.F.B.; Marti, R.K. Open Reduction and Internal Fixation of Three and Four-Part Fractures of the Proximal Part of the Humerus. JBJS 2002, 84, 1919–1925. [Google Scholar] [CrossRef]
- Gerber, C.; Hersche, O.; Berberat, C. The Clinical Relevance of Posttraumatic Avascular Necrosis of the Humeral Head. J. Shoulder Elb. Surg. 1998, 7, 586–590. [Google Scholar] [CrossRef]
- Gomberawalla, M.M.; Miller, B.S.; Coale, R.M.; Bedi, A.; Gagnier, J.J. Meta-Analysis of Joint Preservation versus Arthroplasty for the Treatment of Displaced 3- and 4-Part Fractures of the Proximal Humerus. Injury 2013, 44, 1532–1539. [Google Scholar] [CrossRef]
- Ortho Evidence. No Difference in 12-Mo Functional Outcome between Nail and Plate Fixation for Proximal Humerus Frx. Available online: https://myorthoevidence.com/AceReports/Report/11146 (accessed on 2 May 2022).
- Lopiz, Y.; Garcia-Coiradas, J.; Garcia-Fernandez, C.; Marco, F. Proximal Humerus Nailing: A Randomized Clinical Trial between Curvilinear and Straight Nails. J. Shoulder Elb. Surg. 2014, 23, 369–376. [Google Scholar] [CrossRef]
- Jobin, C.M.; Galdi, B.; Anakwenze, O.A.; Ahmad, C.S.; Levine, W.N. Reverse Shoulder Arthroplasty for the Management of Proximal Humerus Fractures. J. Am. Acad. Orthop. Surg. 2015, 23, 190–201. [Google Scholar] [CrossRef]
- Szeriip, B.W.; Morris, B.J.; Edwards, T.B. Reverse Shoulder Arthroplasty for Trauma: When, Where, and How. Instr. Course Lect. 2016, 65, 10. [Google Scholar]
- Boileau, P.; Chuinard, C.; Le Huec, J.-C.; Walch, G.; Trojani, C. Proximal Humerus Fracture Sequelae: Impact of a New Radiographic Classification on Arthroplasty. Clin. Orthop. Relat. Res. 2006, 442, 121–130. [Google Scholar] [CrossRef]
- Raiss, P.; Edwards, T.B.; Collin, P.; Bruckner, T.; Zeifang, F.; Loew, M.; Boileau, P.; Walch, G. Reverse Shoulder Arthroplasty for Malunions of the Proximal Part of the Humerus (Type-4 Fracture Sequelae). J. Bone Jt. Surg. Am. 2016, 98, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.A.; Tanoira, I.; Ranalletta, M.; Kunze, K.N.; Farivar, D.; Perry, A.; Chahla, J. Cemented vs. Uncemented Reverse Shoulder Arthroplasty for Proximal Humeral Fractures: A Systematic Review and Meta-Analysis. J. Shoulder Elbow. Surg. 2022, 31, e101–e119. [Google Scholar] [CrossRef] [PubMed]
- Formaini, N.T.; Everding, N.G.; Levy, J.C.; Rosas, S. Tuberosity Healing after Reverse Shoulder Arthroplasty for Acute Proximal Humerus Fractures: The “Black and Tan” Technique. J. Shoulder Elbow. Surg. 2015, 24, e299–e306. [Google Scholar] [CrossRef] [PubMed]
- Jo, O.; Borbas, P.; Grubhofer, F.; Ek, E.T.; Pullen, C.; Treseder, T.; Ernstbrunner, L. Prosthesis Designs and Tuberosity Fixation Techniques in Reverse Total Shoulder Arthroplasty: Influence on Tuberosity Healing in Proximal Humerus Fractures. J. Clin. Med. 2021, 10, 4146. [Google Scholar] [CrossRef] [PubMed]
- Gallinet, D.; Ohl, X.; Decroocq, L.; Dib, C.; Valenti, P.; Boileau, P. French Society for Orthopaedic Surgery (SOFCOT) Is Reverse Total Shoulder Arthroplasty More Effective than Hemiarthroplasty for Treating Displaced Proximal Humerus Fractures in Older Adults? A Systematic Review and Meta-Analysis. Orthop. Traumatol. Surg. Res. 2018, 104, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Suroto, H.; De Vega, B.; Deapsari, F.; Prajasari, T.; Wibowo, P.A.; Samijo, S.K. Reverse Total Shoulder Arthroplasty (RTSA) versus Open Reduction and Internal Fixation (ORIF) for Displaced Three-Part or Four-Part Proximal Humeral Fractures: A Systematic Review and Meta-Analysis. EFORT Open Rev. 2021, 6, 941–955. [Google Scholar] [CrossRef]
- Fraser, A.N.; Bjørdal, J.; Wagle, T.M.; Karlberg, A.C.; Lien, O.A.; Eilertsen, L.; Mader, K.; Apold, H.; Larsen, L.B.; Madsen, J.E.; et al. Reverse Shoulder Arthroplasty Is Superior to Plate Fixation at 2 Years for Displaced Proximal Humeral Fractures in the Elderly: A Multicenter Randomized Controlled Trial. J. Bone Jt. Surg. Am. 2020, 102, 477–485. [Google Scholar] [CrossRef]
- Chen, L.; Xing, F.; Xiang, Z. Effectiveness and Safety of Interventions for Treating Adults with Displaced Proximal Humeral Fracture: A Network Meta-Analysis and Systematic Review. PLoS ONE 2016, 11, e0166801. [Google Scholar] [CrossRef]
- Orman, S.; Mohamadi, A.; Serino, J.; Murphy, J.; Hanna, P.; Weaver, M.J.; Dyer, G.; Nazarian, A.; von Keudell, A. Comparison of Surgical and Non-Surgical Treatments for 3- and 4-Part Proximal Humerus Fractures: A Network Meta-Analysis. Shoulder Elb. 2020, 12, 99–108. [Google Scholar] [CrossRef]
- Davey, M.S.; Hurley, E.T.; Anil, U.; Condren, S.; Kearney, J.; O’Tuile, C.; Gaafar, M.; Mullett, H.; Pauzenberger, L. Management Options for Proximal Humerus Fractures—A Systematic Review & Network Meta-Analysis of Randomized Control Trials. Injury 2022, 53, 244–249. [Google Scholar] [CrossRef]
- Cole, P.A. A Word of Caution: Success May Be Limited to 2 Years and Highly Displaced OTA/AO B2 and C2 Injuries: Commentary on an Article by Alexander Nilsskog Fraser, MD; et al.: “Reverse Total Shoulder Arthroplasty Is Superior to Plate Fixation at 2 Years for Displaced Proximal Humeral Fractures in the Elderly. A Multicenter Randomized Controlled Trial.”. J. Bone Jt. Surg. Am. 2020, 102, e30. [Google Scholar] [CrossRef] [PubMed]
- Bacle, G.; Nové-Josserand, L.; Garaud, P.; Walch, G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J. Bone Jt. Surg. 2017, 99, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Slobogean, G.P.; Osterhoff, G.; O’Hara, N.; D’Cruz, J.; Sprague, S.; Bansback, N.; Evaniew, N. A Cost-Effectiveness Analysis of Reverse Total Shoulder Arthroplasty versus Hemiarthroplasty for the Management of Complex Proximal Humeral Fractures in the Elderly. Orthop. Proc. 2016, 98, 8. [Google Scholar] [CrossRef]
- Austin, D.C.; Torchia, M.T.; Tosteson, A.N.A.; Gitajn, I.L.; Tapp, S.J.; Bell, J.-E. The Cost-Effectiveness of Reverse Total Shoulder Arthroplasty Versus Open Reduction Internal Fixation for Proximal Humerus Fractures in the Elderly. Iowa Orthop. J. 2020, 40, 20–29. [Google Scholar]
- Neer, C.S. The Classic: Articular Replacement for the Humeral Head. Clin. Orthop. Relat. Res. 2011, 469, 2409–2421. [Google Scholar] [CrossRef]
- Kontakis, G.; Tosounidis, T.; Galanakis, I.; Megas, P. Prosthetic Replacement for Proximal Humeral Fractures. Injury 2008, 39, 1345–1358. [Google Scholar] [CrossRef]
- Schultz, B.J.; Lowe, D.T.; Egol, K.A.; Zuckerman, J.D. Shoulder Hemiarthroplasty for Proximal Humerus Fracture. J. Orthop. Trauma 2021, 35, S3. [Google Scholar] [CrossRef]
- Laas, N.; Engelsma, Y.; Hagemans, F.J.A.; Hoelen, M.A.; van Deurzen, D.F.P.; Burger, B.J. Reverse or Hemi Shoulder Arthroplasty in Proximal Humerus Fractures: A Single-Blinded Prospective Multicenter Randomized Clinical Trial. J. Orthop. Trauma 2021, 35, 252–258. [Google Scholar] [CrossRef]
- Freeman, T.R.; Dunn, R.H.; Ko, K.J.W.; Seidl, A.J. Hemiarthroplasty for Proximal Humerus Fracture—A Dying Art. Ann. Jt. 2021, 6, 15. [Google Scholar] [CrossRef]
- Wiesel, B.B.; Nagda, S.; Williams, G.R. Technical Pitfalls of Shoulder Hemiarthroplasty for Fracture Management. Orthop. Clin. N. Am. 2013, 44, 317–329, viii. [Google Scholar] [CrossRef]
- Eichenseer, P.; Nowinski, R.; Patel, D. Shoulder Hemiarthroplasty Technique: Shoulder Hemiarthroplasty, Postoperative Rehabilitation, Complications. Available online: https://emedicine.medscape.com/article/2000818-technique#c3 (accessed on 4 May 2022).
- Aldinger, P.R.; Raiss, P.; Rickert, M.; Loew, M. Complications in Shoulder Arthroplasty: An Analysis of 485 Cases. Int. Orthop. 2010, 34, 517–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kontakis, G.; Koutras, C.; Tosounidis, T.; Giannoudis, P. Early Management of Proximal Humeral Fractures with Hemiarthroplasty: A Systematic Review. J. Bone Jt. Surg. Br. 2008, 90, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Postacchini, R.; Castagna, A.; Borroni, M.; Cinotti, G.; Postacchini, F.; Gumina, S. Total Shoulder Arthroplasty for the Treatment of Failed Hemiarthroplasty in Patients with Fracture of the Proximal Humerus. J. Shoulder Elbow. Surg. 2012, 21, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Krishnan, S.G.; Tinsi, L.; Walch, G.; Coste, J.S.; Molé, D. Tuberosity Malposition and Migration: Reasons for Poor Outcomes after Hemiarthroplasty for Displaced Fractures of the Proximal Humerus. J. Shoulder Elb. Surg. 2002, 11, 401–412. [Google Scholar] [CrossRef]
- Boons, H.W.; Goosen, J.H.; van Grinsven, S.; van Susante, J.L.; van Loon, C.J. Hemiarthroplasty for Humeral Four-Part Fractures for Patients 65 Years and Older: A Randomized Controlled Trial. Clin. Orthop. Relat. Res. 2012, 470, 3483–3491. [Google Scholar] [CrossRef]
- Solberg, B.; Moon, C.; Franco, D.; Paiement, G. Surgical Treatment of Three and Four-Part Proximal Humeral F. J. Bone Jt. Surg. Am. 2009, 91, 1689–1697. [Google Scholar] [CrossRef]
- Antuña, S.A.; Sperling, J.W.; Cofield, R.H. Shoulder Hemiarthroplasty for Acute Fractures of the Proximal Humerus: A Minimum Five-Year Follow-Up. J. Shoulder Elb. Surg. 2008, 17, 202–209. [Google Scholar] [CrossRef]
- Hoel, S.; Jensen, T.G.; Falster, O.; Ulstrup, A. Hemiarthroplasty for Proximal Humerus Fracture and Consequences of a Comminuted Greater Tubercle Fragment. Musculoskelet Surg. 2016, 100, 9–14. [Google Scholar] [CrossRef]



| 10 Year Implant Survival | 87–93% |
| 15 Year Implant Survival | 79–92% |
| Constant Score | 57–62% |
| Forward Flexion | 101–139° |
| Abduction | 86–135° |
| External Rotation | 11–48° |
| Prosthetic Joint Infection | 2.4% |
| Grade III/IV Scapular Notching | 29.4% |
| Acromial/Scapular Fracture | 2.6% |
| Loosening/Instability | 1.3% |
| Operative Method | Advantages | Disadvantages |
|---|---|---|
| Closed Reduction Percutaneous Pinning |
|
|
| Open Reduction Internal Fixation |
|
|
| Intramedullary nailing |
|
|
| Reverse Total Shoulder Arthroplasty |
|
|
| Hemi Arthroplasty |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, H.P.; Gutbrod, J.; Strelzow, J.A.; Maassen, N.H.; Shi, L. Management of Proximal Humerus Fractures in Adults—A Scoping Review. J. Clin. Med. 2022, 11, 6140. https://doi.org/10.3390/jcm11206140
Baker HP, Gutbrod J, Strelzow JA, Maassen NH, Shi L. Management of Proximal Humerus Fractures in Adults—A Scoping Review. Journal of Clinical Medicine. 2022; 11(20):6140. https://doi.org/10.3390/jcm11206140
Chicago/Turabian StyleBaker, Hayden P., Joseph Gutbrod, Jason A. Strelzow, Nicholas H. Maassen, and Lewis Shi. 2022. "Management of Proximal Humerus Fractures in Adults—A Scoping Review" Journal of Clinical Medicine 11, no. 20: 6140. https://doi.org/10.3390/jcm11206140
APA StyleBaker, H. P., Gutbrod, J., Strelzow, J. A., Maassen, N. H., & Shi, L. (2022). Management of Proximal Humerus Fractures in Adults—A Scoping Review. Journal of Clinical Medicine, 11(20), 6140. https://doi.org/10.3390/jcm11206140







