Caspase-1 Inhibition Reduces Occurrence of PANoptosis in Macrophages Infected by E. faecalis OG1RF
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Enterococcus faecalis and Macrophages
2.2. RAW264.7 Cells Infected with E. faecalis
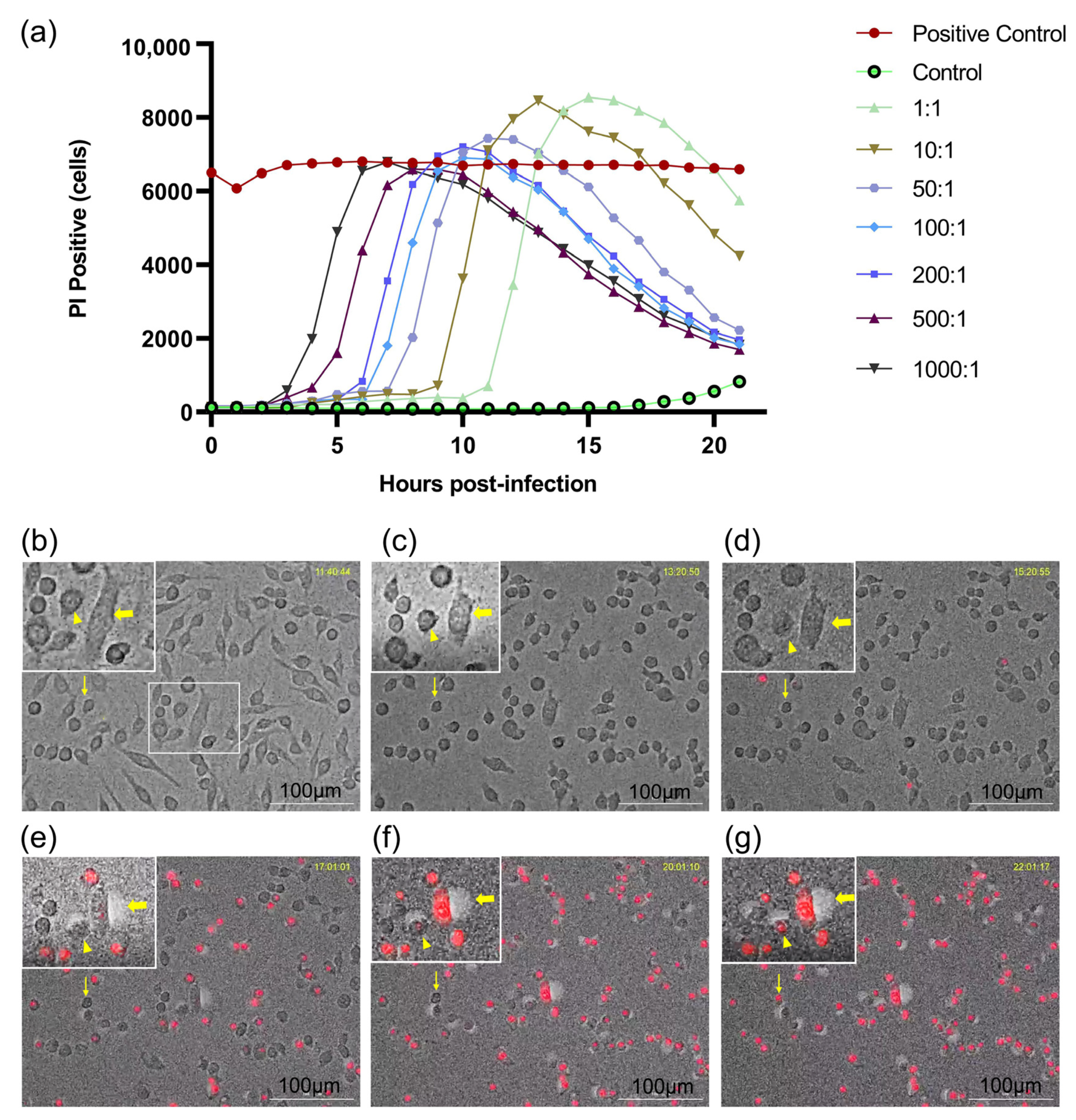
2.3. Real-Time Cell Death Analysis
2.4. Quantitative Real-time Polymerase Chain Reaction (RT-qPCR)
2.5. Western Blotting
2.6. Scanning Electron Microscopy
2.7. Statistical Analysis
3. Results
3.1. Dynamic Changes in Macrophages Infected with E. faecalis in a Dose-Dependent Manner
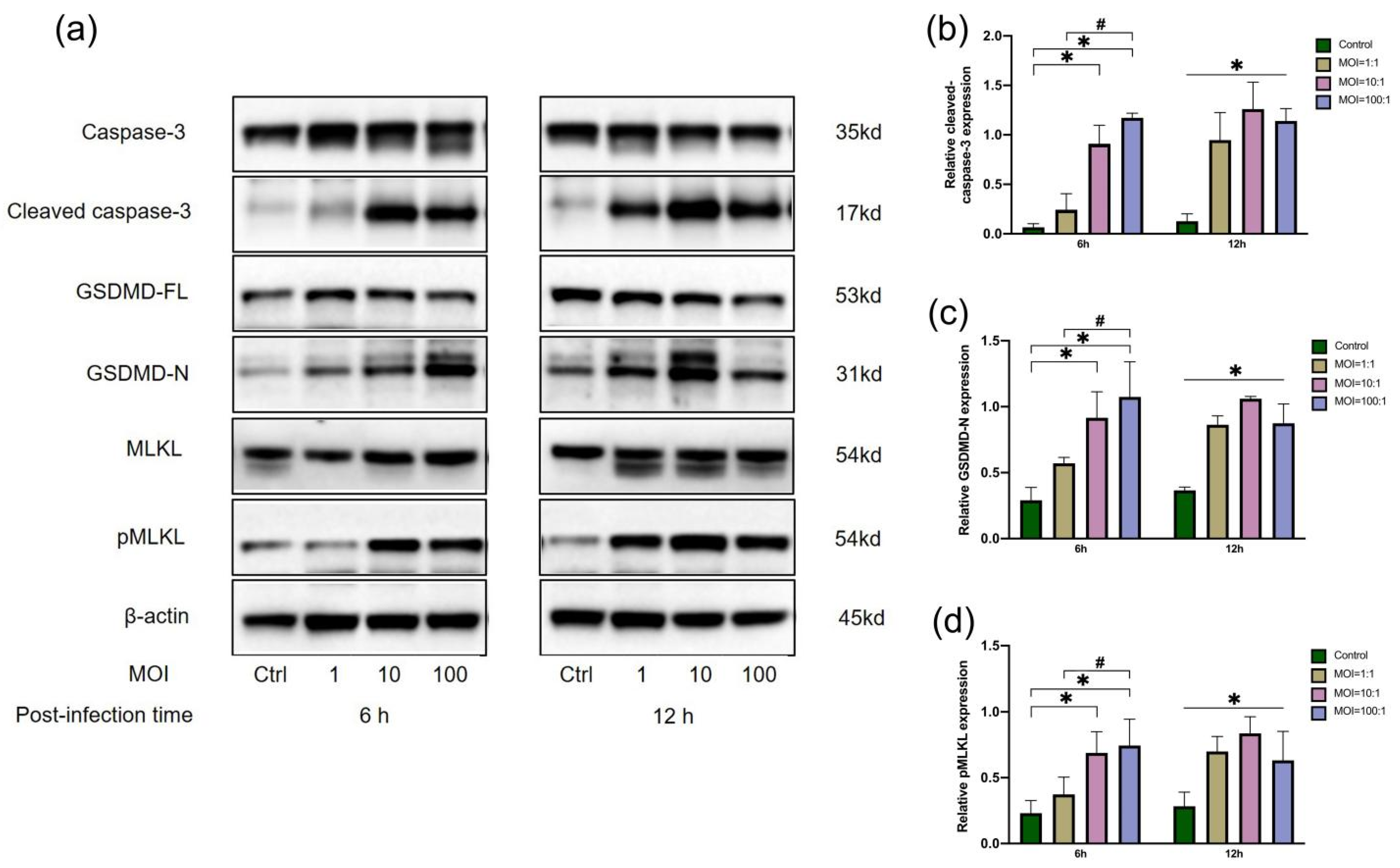
3.2. Protein Expression of Macrophages Infected by E. faecalis at Three MOIs
3.3. The Effect of Caspase-1 Inhibitor on Real-Time Cell Death of Macrophages
3.4. The Effect of Caspase-1 Inhibitor on Gene Expression of Two Cytokines
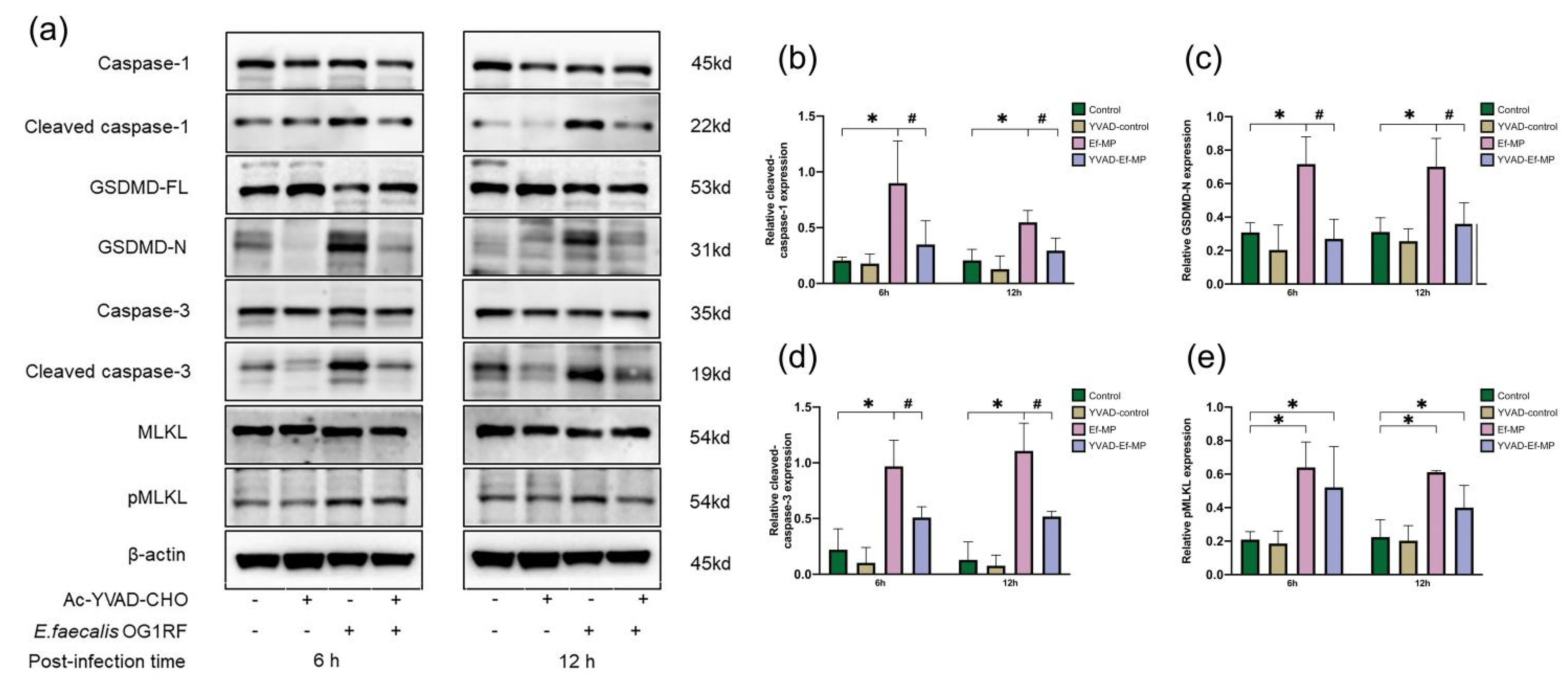
3.5. Protein Expression of E. faecalis-Infected Macrophages Treated with a Caspase-1 Inhibitor
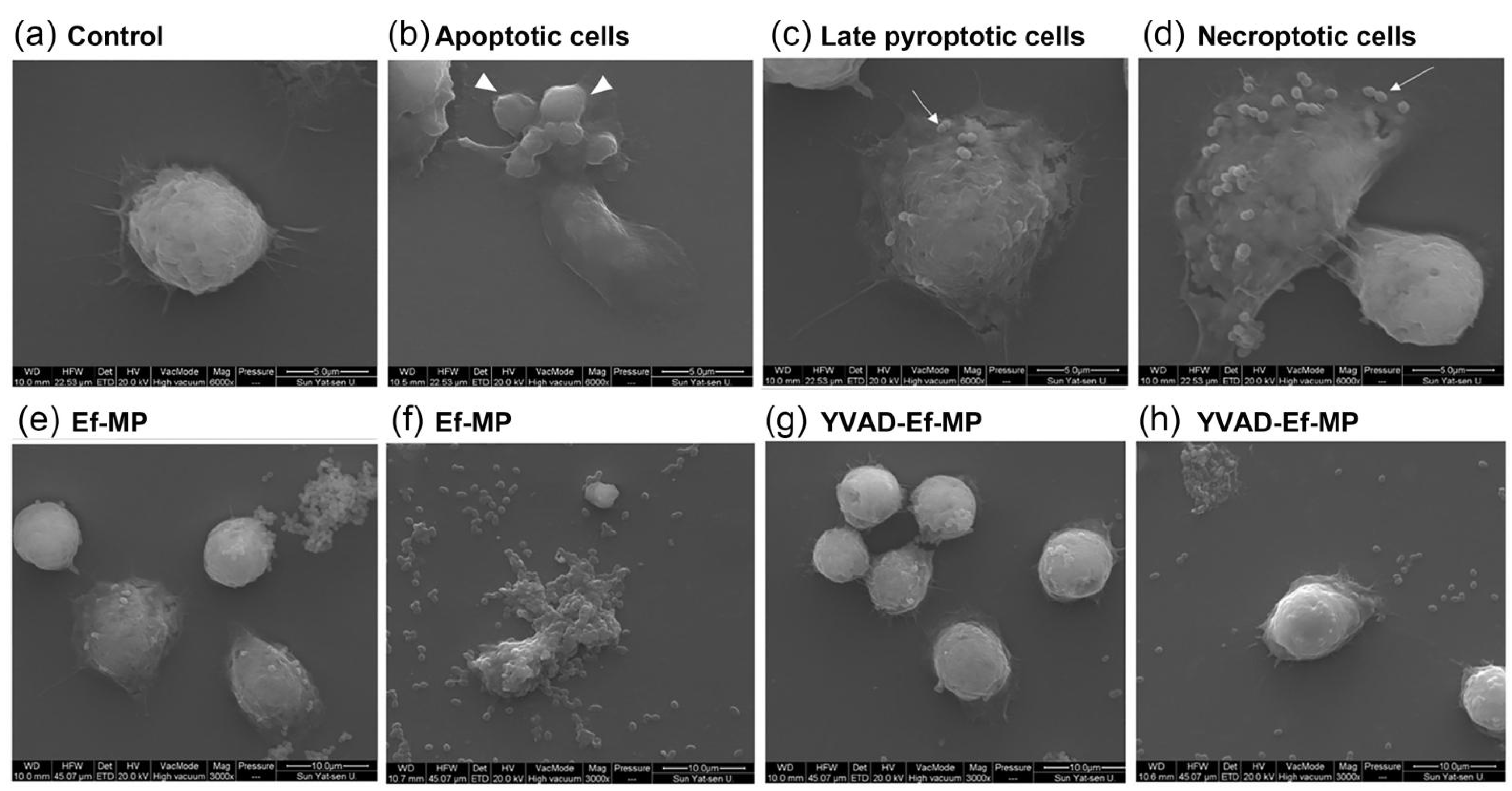
3.6. Morphological Modification of E. faecalis-Infected Macrophages under Caspase-1 Inhibitor Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, B.E. The life and times of the Enterococcus. Clin. Microbiol. Rev. 1990, 3, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Elashiry, M.M.; Tian, F.; Elashiry, M.; Zeitoun, R.; Elsayed, R.; Andrews, M.L.; Bergeon, B.E.; Cutler, C.; Tay, F. Enterococcus faecalis shifts macrophage polarization toward M1-like phenotype with an altered cytokine profile. J. Oral. Microbiol. 2021, 13, 1868152. [Google Scholar] [CrossRef] [PubMed]
- Kayaoglu, G.; Orstavik, D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit. Rev. Oral. Biol. Med. 2004, 15, 308–320. [Google Scholar] [CrossRef] [Green Version]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef]
- Gomes, B.P.F.A.; Pinheiro, E.; Gade-Neto, C.R.; Sousa, E.L.R.; Ferraz, C.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. Microbiological examination of infected dental root canals. Oral. Microbiol. Immunol. 2004, 19, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Gomes, B.P.; Pinheiro, E.; Sousa, E.L.; Jacinto, R.; Zaia, A.A.; Ferraz, C.; de Souza-Filho, F.J. Enterococcus faecalis in dental root canals detected by culture and by polymerase chain reaction analysis. Oral Surg. Oral. Med. Oral. Pathol. Oral Radiol. Endod. 2006, 102, 247–253. [Google Scholar] [CrossRef]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef] [Green Version]
- Sakko, M.; Tjäderhane, L.; Rautemaa-Richardson, R. Microbiology of Root Canal Infections. Prim. Dent. J. 2016, 5, 84–89. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Zhang, C.; Du, J.; Peng, Z. Correlation between Enterococcus faecalis and Persistent Intraradicular Infection Compared with Primary Intraradicular Infection: A Systematic Review. J. Endod. 2015, 41, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Place, D.E.; Lee, S.; Kanneganti, T.-D. PANoptosis in microbial infection. Curr. Opin. Microbiol. 2020, 59, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.S.; Tweedell, R.E.; Kanneganti, T.-D. PANoptosis components, regulation, and implications. Aging 2020, 12, 11163–11164. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, T.; Kanneganti, T.D. Pyroptosis in Antiviral Immunity. Curr. Top. Microbiol. Immunol. 2019. Online ahead of print. [Google Scholar] [CrossRef]
- Banoth, B.; Tuladhar, S.; Karki, R.; Sharma, B.R.; Briard, B.; Kesavardhana, S.; Burton, A.; Kanneganti, T.-D. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J. Biol. Chem. 2020, 295, 18276–18283. [Google Scholar] [CrossRef]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Lee, E.; Banoth, B.; Malireddi, R.S.; Samir, P.; Tuladhar, S.; Mummareddy, H.; Burton, A.R.; Vogel, P.; et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight. 2020, 5, e136720. [Google Scholar] [CrossRef]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.S.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.-D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef] [Green Version]
- Lukens, J.; Gurung, P.; Vogel, P.; Johnson, G.R.; Carter, R.A.; McGoldrick, D.J.; Bandi, S.; Calabrese, C.R.; Walle, L.V.; Lamkanfi, M.; et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 2014, 516, 246–249. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Williams, E.P.; Malireddi, R.K.S.; Karki, R.; Banoth, B.; Burton, A.; Webby, R.; Channappanavar, R.; Jonsson, C.B.; Kanneganti, T.-D. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J. Biol. Chem. 2020, 295, 14040–14052. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseasses. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Malireddi, R.; Gurung, P.; Mavuluri, J.; Dasari, T.K.; Klco, J.M.; Chi, H.; Kanneganti, T.-D. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J. Exp. Med. 2018, 215, 1023–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malireddi, R.; Kesavardhana, S.; Kanneganti, T.-D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front. Cell Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155 (Pt 6), 1749–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, K.K.L.; Tay, W.H.; Janela, B.; Yong, A.M.H.; Liew, T.H.; Madden, L.; Keogh, D.; Barkham, T.M.S.; Ginhoux, F.; Becker, D.L.; et al. Enterococcus faecalis Modulates Immune Activation and Slows Healing During Wound Infection. J. Infect. Dis. 2017, 216, 1644–1654. [Google Scholar] [CrossRef] [Green Version]
- Chi, D.; Lin, X.; Meng, Q.; Tan, J.; Gong, Q.; Tong, Z. Real-Time Induction of Macrophage Apoptosis, Pyroptosis, and Necroptosis by Enterococcus faecalis OG1RF and Two Root Canal Isolated Strains. Front. Cell Infect. Microbiol. 2021, 11, 720147. [Google Scholar] [CrossRef]
- Gentry-Weeks, C.R.; Karkhoff-Schweizer, R.; Pikis, A.; Estay, M.; Keith, J.M. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect. Immun. 1999, 67, 2160–2165. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Keitany, G.; Li, Y.; Wang, Y.; Ball, H.L.; Goldsmith, E.J.; Orth, K. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 2006, 312, 1211–1214. [Google Scholar] [CrossRef] [Green Version]
- Orning, P.; Weng, D.; Starheim, K.; Ratner, D.; Best, Z.; Lee, B.; Brooks, A.; Xia, S.; Wu, H.; Kelliher, M.A.; et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 2018, 362, 1064–1069. [Google Scholar] [CrossRef]
- Paquette, N.; Conlon, J.; Sweet, C.; Rus, F.; Wilson, L.; Pereira, A.; Rosadini, C.V.; Goutagny, N.; Weber, A.N.R.; Lane, W.S.; et al. Serine/threonine acetylation of TGFbeta-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 12710–12715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, N.H.; Dillon, C.P.; Snyder, A.G.; Fitzgerald, P.; Wynosky-Dolfi, M.A.; Zwack, E.E.; Hu, B.; Fitzgerald, L.; Mauldin, E.A.; Copenhaver, A.M.; et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7385–7390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjo, H.; Nakayama, J.; Yoshizawa, T.; Fehling, H.J.; Akira, S.; Taki, S. Cutting Edge: TAK1 Safeguards Macrophages against Proinflammatory Cell Death. J. Immunol. 2019, 203, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Kulild, J. Master apical file size—Smaller or larger: A systematic review of microbial reduction. Int. Endod. J. 2015, 48, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Shankar, N. Enterococcus faecalis infection activates phosphatidylinositol 3-kinase signaling to block apoptotic cell death in macrophages. Infect. Immun. 2014, 82, 5132–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.-D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, N.M.; Lamkanfi, M. Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis. Cold Spring Harb. Perspect. Biol. 2020, 12, a036392. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Y.; Wu, Y.; Liu, Y.; Chen, K.; Liu, X.; Zou, Z.; Huang, X.; Wu, M. Triggering Receptors Expressed on Myeloid Cells 2 Promotes Corneal Resistance Against Pseudomonas aeruginosa by Inhibiting Caspase-1-Dependent Pyroptosis. Front. Immunol. 2018, 9, 1121. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Cao, R.; Hu, H.-M. TLR and NLRP3 inflammasome-dependent innate immune responses to tumor-derived autophagosomes (DRibbles). Cell Death Dis. 2016, 7, e2322. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Wu, J.; Li, Y.; Xia, Y.; Chu, P.; Qi, K.; Yan, Z.; Yao, H.; Liu, Y.; Xu, K.; et al. Blockage of caspase-1 activation ameliorates bone marrow inflammation in mice after hematopoietic stem cell transplantation. Clin. Immunol. 2016, 162, 84–90. [Google Scholar] [CrossRef]
- Fei, X.-F.; Wang, B.-X.; Tashiro, S.; Li, T.-J.; Ma, J.-S.; Ikejima, T. Apoptotic effects of ginsenoside Rh2 on human malignant melanoma A375-S2 cells. Acta Pharmacol. Sin. 2002, 23, 315–322. [Google Scholar] [PubMed]
- Wu, Z.; Wu, L.-J.; Li, L.-H.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Shikonin regulates HeLa cell death via caspase-3 activation and blockage of DNA synthesis. J. Asian Nat. Prod. Res. 2004, 6, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-D.; Pan, P.-H.; Liu, B.; Su, X.-L.; Zhang, L.-M.; Tan, H.-Y.; Cao, Z.; Zhou, Z.-R.; Li, H.-T.; Li, H.-S.; et al. Inhibition of Alveolar Macrophage Pyroptosis Reduces Lipopolysaccharide-induced Acute Lung Injury in Mice. Chin. Med. J. 2015, 128, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, W.-T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, D.; Zhang, Y.; Lin, X.; Gong, Q.; Tong, Z. Caspase-1 Inhibition Reduces Occurrence of PANoptosis in Macrophages Infected by E. faecalis OG1RF. J. Clin. Med. 2022, 11, 6204. https://doi.org/10.3390/jcm11206204
Chi D, Zhang Y, Lin X, Gong Q, Tong Z. Caspase-1 Inhibition Reduces Occurrence of PANoptosis in Macrophages Infected by E. faecalis OG1RF. Journal of Clinical Medicine. 2022; 11(20):6204. https://doi.org/10.3390/jcm11206204
Chicago/Turabian StyleChi, Danlu, Yuejiao Zhang, Xinwei Lin, Qimei Gong, and Zhongchun Tong. 2022. "Caspase-1 Inhibition Reduces Occurrence of PANoptosis in Macrophages Infected by E. faecalis OG1RF" Journal of Clinical Medicine 11, no. 20: 6204. https://doi.org/10.3390/jcm11206204
APA StyleChi, D., Zhang, Y., Lin, X., Gong, Q., & Tong, Z. (2022). Caspase-1 Inhibition Reduces Occurrence of PANoptosis in Macrophages Infected by E. faecalis OG1RF. Journal of Clinical Medicine, 11(20), 6204. https://doi.org/10.3390/jcm11206204





