SARS-CoV-2—The Role of Natural Immunity: A Narrative Review
Abstract
:1. Introduction
2. Aims
3. Material and Methods
4. Results
4.1. Duration and Type of Immunity from Previous SARS-CoV-2 Infection
4.2. Cellular Immunity
4.3. Cross-Reactivity
4.4. The Duration of Post-Vaccination Immune Protection
4.5. Probability of Reinfection in the Recovered Subjects, and Its Clinical Manifestations
4.6. Comparisons between Vaccinated and Unvaccinated Subjects in the Development of Immunity and Therefore of Possible Reinfections
4.7. The role of Hybrid Immunity
4.8. Effectiveness of Natural and Artificial Immunity against Omicron
4.9. Incidence of Adverse Effects after Vaccination in the Recovered Compared to COVID-19-Naïve Subjects
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diani, S. The Coronavirus Pandemic—A Systemic Overview. Preprints 2020. [Google Scholar] [CrossRef]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Maddox, T.; Lorenzi, L.; Studley, R.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat. Commun. 2021, 12, 1–6250. [Google Scholar] [CrossRef] [PubMed]
- Petráš, M. Highly effective naturally acquired protection against COVID-19 persists for at least 1 year: A meta-analysis. J. Am. Med. Dir. Assoc. 2021, 22, 2263–2265. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Diani, S. Are Diseases the Best Possible Response of the Complex Living System to Stimuli? Int. J. Hist. Philos. Med. 2018, 8, 10802. [Google Scholar]
- Diani, S. A new model for chronic diseases. J. Med. Hypotheses 2018, 113, 30–39. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef]
- Harvey, R.; Rassen, J.; Kabelac, C.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A.; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Real-world data suggest anti- body positivity to SARS-CoV-2 is associated with a decreased risk of future infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- Houlihan, C.F.; Vora, N.; Byrne, T.; Lewer, D.; Kelly, G.; Heaney, J.; Gandhi, S.; Spyer, M.J.; Beale, R.; Cherepanov, P.; et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet 2020, 396, e6–e7. [Google Scholar] [CrossRef]
- Hanrath, A.T.; Payne, B.A.I.; Duncan, C.J.A. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J. Infect. 2020, 82, e29–e30. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Stellas, D.; Rosati, M.; Sergentanis, T.N.; Hu, X.; Politou, M.; Pappa, V.; Ntanasis-Stathopoulos, I.; Karaliota, S.; Bear, J.; et al. SARS-CoV-2 antibody kinetics eight months from COVID-19 onset: Persistence of spike antibodies but loss of neutralizing antibodies in 24% of convalescent plasma donors. Eur. J. Intern. Med. 2021, 89, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.P.; Prévost, J.; Nayrac, M.; Beaudoin-Bussières, G.; Benlarbi, M.; Gasser, R.; Brassard, N.; Laumaea, A.; Gong, S.Y.; Bourassa, C.; et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep. Med. 2021, 2, 100290. [Google Scholar] [CrossRef] [PubMed]
- Gussarow, D.; Bonifacius, A.; Cossmann, A.; Stankov, M.V.; Mausberg, P.; Tischer-Zimmermann, S.; Gödecke, N.; Kalinke, U.; Behrens, G.M.N.; Blasczyk, R.; et al. Long-Lasting Immunity Against SARS-CoV-2: Dream or Reality? Front. Med. 2021, 8, 770381. [Google Scholar] [CrossRef]
- Petersen, M.S.; Hansen, C.B.; Kristiansen, M.F.; Fjallsbak, J.P.; Larsen, S.; Hansen, J.L.; Jarlhelt, I.; Pérez-Alós, L.; Steig, B.; Christiansen, D.H.; et al. SARS-CoV-2 Natural Antibody Response Persists for at Least 12 Months in a Nationwide Study From the Faroe Islands. Open Forum. Infect. Dis. 2021, 8, ofab378. [Google Scholar] [CrossRef]
- Haveri, A.; Ekström, N.; Solastie, A.; Virta, C.; Österlund, P.; Isosaari, E.; Nohynek, H.; A Palmu, A.; Melin, M. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection in humans. Eur. J. Immunol. 2021, 51, 3202–3213. [Google Scholar] [CrossRef]
- Alfegob, D.; Sullivan, A.; Poirier, B.; Williams, J.; Grover, A.; Gillim, L.; Adcock, D.; Letovsky, S. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalMedicine 2021, 36, 100902. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Peng, Y.; Liang, Y.; Wei, J.; Xing, L.; Guo, L.; Li, X.; Li, J.; Wang, J.; et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat. Microbiol. 2022, 7, 423–433. [Google Scholar] [CrossRef]
- Alejo, J.L.; Mitchell, J.; Chang, A.; Chiang, T.P.Y.; Massie, A.B.; Segev, D.L.; Makary, M.A. Prevalence and Durability of SARS-CoV-2 Antibodies Among Unvaccinated US Adults by History of COVID-19. JAMA 2022, 327, 1085–1087. [Google Scholar] [CrossRef]
- De Giorgi, V.; West, K.A.; Henning, A.N.; Chen, L.N.; Holbrook, M.R.; Gross, R.; Liang, J.; Postnikova, E.; Trenbeath, J.; Pogue, S.; et al. Naturally Acquired SARS-CoV-2 Immunity Persists for Up to 11 Months Following Infection. J. Infect. Dis. 2021, 224, 1294–1304. [Google Scholar] [CrossRef]
- Ni, L.; Ye, F.; Cheng, M.-L.; Feng, Y.; Deng, Y.-Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977. [Google Scholar] [CrossRef]
- Ng, O.-W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.-J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef]
- Radbruch, A.; Chang, H.D. A long-term perspective on immunity to COVID. Nature 2021, 595, 359–360. [Google Scholar] [CrossRef]
- Rank, A.; Tzortzini, A.; Kling, E.; Schmid, C.; Claus, R.; Löll, E.; Burger, R.; Römmele, C.; Dhillon, C.; Müller, K.; et al. One Year after Mild COVID-19: The Majority of Patients Maintain Specific Immunity, But One in Four Still Suffer from Long-Term Symptoms. J. Clin. Med. 2021, 10, 3305. [Google Scholar] [CrossRef]
- Ripperger, T.J.; Uhrlaub, J.L.; Watanabe, M.; Wong, R.; Castaneda, Y.; Pizzato, H.A.; Thompson, M.R.; Bradshaw, C.; Weinkauf, C.C.; Bime, C.; et al. Orthogonal SARS-CoV-2 Serological Assays Enable Surveillance of Low-Prevalence Communities and Reveal Durable Humoral Immunity. Immunity 2020, 53, 925–933. [Google Scholar] [CrossRef]
- Yao, L.; Wang, G.-L.; Shen, Y.; Wang, Z.-Y.; Zhan, B.-D.; Duan, L.-J.; Lu, B.; Shi, C.; Gao, Y.-M.; Peng, H.-H.; et al. Persistence of Antibody and Cellular Immune Responses in Coronavirus Disease 2019 Patients Over Nine Months After Infection. J. Infect. Dis. 2021, 224, 586–594. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Vibholm, L.K.; Monrad, I.; Olesen, R.; Frattari, G.S.; Pahus, M.H.; Højen, J.F.; Gunst, J.D.; Erikstrup, C.; Holleufer, A.; et al. SARS-CoV-2 elicits robust adaptive immune responses regardless of disease severity. EBioMedicine 2021, 68, 103410. [Google Scholar] [CrossRef]
- Dehgani-Mobaraki, P.; Zaidi, A.K.; Yadav, N.; Floridi, A.; Floridi, E. Longitudinal observation of antibody responses for 14 months after SARS-CoV-2 infection. Clin. Immunol. 2021, 230, 108814. [Google Scholar] [CrossRef]
- Sherina, N.; Piralla, A.; Du, L.; Wan, H.; Kumagai-Braesch, M.; Andréll, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Medicines 2021, 2, 281–295. [Google Scholar] [CrossRef]
- Chivese, T.; Matizanadzo, J.T.; Musa, O.A.H.; Hindy, G.; Furuya-Kanamori, L.; Islam, N.; Al-Shebly, R.; Shalaby, R.; Habibullah, M.; Al-Marwani, T.A.; et al. The prevalence of adaptive immunity to COVID-19 and reinfection after recovery—A comprehensive systematic review and meta-analysis. Pathog. Glob. Health 2022, 116, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.-H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, X.; Xin, Q.; Pan, Y.; Hu, Y.; Li, J.; Chu, Y.; Feng, Y.; Wang, Q. Neutralizing Antibody Responses to Severe Acute Respiratory Syndrome Coronavirus 2 in Coronavirus Disease 2019 In patients and Convalescent Patients. Clin. Infect. Dis. 2020, 71, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Redd, A.D.; Nardin, A.; Kared, H.; Bloch, E.M.; Pekosz, A.; Laeyendecker, O.; Abel, B.; Fehlings, M.; Quinn, T.C.; Tobian, A.A. CD8+ T cell responses in COVID-19 convalescent individuals target conserved epitopes from multiple prominent SARS-CoV-2 circulating variants. Open Forum. Infect. Dis. 2021, 8, ofab143. [Google Scholar] [CrossRef]
- Poon, M.M.; Rybkina, K.; Kato, Y.; Kubota, M.; Matsumoto, R.; Bloom, N.I.; Zhang, Z.; Hastie, K.M.; Grifoni, A.; Weiskopf, D.; et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol. 2021, 6, eabl9105. [Google Scholar] [CrossRef]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef]
- Sui, Y.; Bekele, Y.; Berzofsky, J.A. Potential SARS-CoV-2 Immune Correlates of Protection in Infection and Vaccine Immunization. Pathogens 2021, 10, 138. [Google Scholar] [CrossRef]
- Greenbaum, J.A.; Kotturi, M.F.; Kim, Y.; Oseroff, C.; Vaughan, K.; Salimi, N.; Vita, R.; Ponomarenko, J.; Scheuermann, R.H.; Sette, A.; et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. USA 2009, 106, 20365–20370. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef]
- Wilkinson, T.M.; Li, C.K.F.; Chui, C.S.C.; Huang, A.K.Y.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.S.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef]
- Smetana, J.; Chlibek, R.; Hanovcova, I.; Sosovickova, R.; Smetanova, L.; Gal, P.; Dite, P. Decreasing Seroprevalence of Measles Antibodies after Vaccination—Possible Gap in Measles Protection in Adults in the Czech Republic. PLoS ONE 2017, 12, e0170257. [Google Scholar] [CrossRef] [Green Version]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Antunes, R.S.; Moore, E.; Rubiro, P.; et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021, 2, 100204. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Pre-existing immunity to SARS-CoV-2: The knowns and unknowns. Nat. Rev. Immunol. 2020, 20, 457–458. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585-15. [Google Scholar] [CrossRef]
- Lucas, C.; Klein, J.; Sundaram, M.E.; Liu, F.; Wong, P.; Silva, J.; Mao, T.; Oh, J.E.; Mohanty, S.; Huang, J.; et al. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat. Med. 2021, 27, 1178–1186. [Google Scholar] [CrossRef]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Ni Chia, W.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021, 53, 108728-13. [Google Scholar] [CrossRef]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Schulien, I.; Kemming, J.; Oberhardt, V.; Wild, K.; Seidel, L.M.; Killmer, S.; Daul, F.; Lago, M.S.; Decker, A.; Luxenburger, H.; et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat. Med. 2020, 181, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Tan, H.X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Kared, H.; Redd, A.D.; Bloch, E.M.; Bonny, T.S.; Sumatoh, H.R.; Kairi, F.; Carbajo, D.; Abel, B.; Newell, E.W.; Bettinotti, M.P.; et al. SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J. Clin. Investig. 2021, 131, e145476. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-N.; Liu, P.-P.; Li, X.-G.; Zhou, S.-J.; Li, H.; Wang, Z.-Y.; Shen, F.; Lu, B.-C.; Long, Y.; Xiao, X.; et al. Neutralizing Antibodies and Cellular Immune Responses Against SARS-CoV-2 Sustained One and a Half Years After Natural Infection. Front. Microbiol. 2022, 12, 803031. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Breton, G.; Mendoza, P.; Hägglöf, T.; Oliveira, T.Y.; Schaefer-Babajew, D.; Gaebler, C.; Turroja, M.; Hurley, A.; Caskey, M.; Nussenzweig, M.C. Persistent cellular immunity to SARS-CoV-2 infection. J. Exp. Med. 2021, 218, e20202515. [Google Scholar] [CrossRef]
- Le Bert, N.; Clapham, H.E.; Tan, A.T.; Ni Chia, W.; Tham, C.Y.; Lim, J.M.; Kunasegaran, K.; Tan, L.W.L.; Dutertre, C.-A.; Shankar, N.; et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 2021, 218, e20202617. [Google Scholar] [CrossRef]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 2021, 184, 169–183. [Google Scholar] [CrossRef]
- Jung, J.H.; Rha, M.-S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.-H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]
- Bonifacius, A.; Tischer-Zimmermann, S.; Dragon, A.C.; Gussarow, D.; Vogel, A.; Krettek, U.; Gödecke, N.; Yilmaz, M.; Kraft, A.R.; Hoeper, M.M.; et al. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity 2021, 54, 340–354. [Google Scholar] [CrossRef]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef]
- Callaway, E. Had COVID? You’ll probably make antibodies for a lifetime. Nature 2021. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Burton, A.R.; Lens, S.; Rees-Spear, C.; Davies, J.; Patel, M.; Gopal, R.; Muir, L.; Aiano, F.; Doores, K.J.; et al. SARS-CoV-2-specific memory B cells can persist in the elderly who have lost detectable neutralizing antibodies. J. Clin. Investig. 2022, 132, e152042. [Google Scholar] [CrossRef]
- ECDC. Assessment of the Further Emergence of the SARS-CoV-2 Omicron VOC in the Context of the Ongoing Delta VOC Transmission in the EU/EEA, 18th Update (2021). Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-assessment-further-emergence-omicron-18th-risk-assessment (accessed on 3 April 2022).
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Rössler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N. Engl. J. Med. 2022, 386, 698–700. [Google Scholar] [CrossRef]
- Mazzoni, A.; Vanni, A.; Spinicci, M.; Capone, M.; Lamacchia, G.; Salvati, L.; Coppi, M.; Antonelli, A.; Carnasciali, A.; Farahvachi, P.; et al. SARS-CoV-2 Spike-Specific CD4+ T Cell Response Is Conserved Against Variants of Concern, Including Omicron. Front. Immunol. 2022, 13, 801431. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4 + and CD8 + T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Ferretti, A.P.; Kula, T.; Wang, Y.; Nguyen, D.M.; Weinheimer, A.; Dunlap, G.S.; Xu, Q.; Nabilsi, N.; Perullo, C.R.; Cristofaro, A.W.; et al. Unbiased Screens Show CD8+ T Cells of COVID-19 Patients Recognize Shared Epitopes in SARS-CoV-2 that Largely Reside outside the Spike Protein. Immunity 2020, 53, 1095–1107.e3. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lübke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.W.; Chen, Y.; Venezia, O.L.; Majerus, R.M.; Shin, D.S.; Carrington, M.N.; Yu, X.G.; Wesemann, D.R.; Moon, J.J.; Luster, A.D.; et al. SARS-CoV-2 epitope-specific CD4+ memory T cell responses across COVID-19 disease severity and antibody durability. Sci. Immunol. 2022, 7, eabl9464. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Selin, L.K. No one is naive: The significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2002, 2, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 521–524. [Google Scholar]
- Yu, X.; Tsibane, T.; McGraw, P.A.; House, F.S.; Keefer, C.J.; Hicar, M.D.; Tumpey, T.M.; Pappas, C.; Perrone, L.A.; Martinez, O.; et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors [published correction appears in Nature. Nature 2008, 455, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Doshi, P. Covid-19: Do many people have pre-existing immunity? BMJ 2020, 370, m3563. [Google Scholar] [CrossRef]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Lineburg, K.E.; Grant, E.J.; Swaminathan, S.; Chatzileontiadou, D.S.; Szeto, C.; Sloane, H.; Panikkar, A.; Raju, J.; Crooks, P.; Rehan, S.; et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity 2021, 54, 1055–1065. [Google Scholar] [CrossRef]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; Van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; Van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef]
- Aydillo, T.; Rombauts, A.; Stadlbauer, D.; Aslam, S.; Abelenda-Alonso, G.; Escalera, A.; Amanat, F.; Jiang, K.; Krammer, F.; Carratala, J.; et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat. Commun. 2021, 12, 3781. [Google Scholar] [CrossRef]
- Mahajan, S.; Kode, V.; Bhojak, K.; Karunakaran, C.; Lee, K.; Manoharan, M.; Ramesh, A.; Hv, S.; Srivastava, A.; Sathian, R.; et al. Immunodominant T-cell epitopes from the SARS-CoV-2 spike antigen reveal robust pre-existing T-cell immunity in unexposed individuals. Sci. Rep. 2021, 11, 13164. [Google Scholar] [CrossRef]
- Wrammert, J.; Koutsonanos, D.; Li, G.-M.; Edupuganti, S.; Sui, J.; Morrissey, M.; McCausland, M.; Skountzou, I.; Hornig, M.; Lipkin, W.I.; et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011, 208, 181–193. [Google Scholar] [CrossRef]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef]
- Majdoubi, A.; Michalski, C.; O’Connell, S.E.; Dada, S.; Narpala, S.; Gelinas, J.; Mehta, D.; Cheung, C.; Winkler, D.F.; Basappa, M.; et al. A majority of uninfected adults show preexisting antibody reactivity against SARS-CoV-2. JCI Insight 2021, 6, e146316. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Westerhuis, B.M. Homologous and heterologous antibodies to coronavirus 229E, NL63, OC43, HKU1, SARS, MERS and SARS-CoV-2 antigens in an age stratified cross-sectional serosurvey in a large tertiary hospital in The Netherlands Preprint at. medRxiv 2020. [Google Scholar] [CrossRef]
- Abela, I.A.; Pasin, C.; Schwarzmüller, M.; Epp, S.; Sickmann, M.E.; Schanz, M.M.; Rusert, P.; Weber, J.; Schmutz, S.; Audigé, A.; et al. Multifactorial seroprofiling dissects the contribution of pre-existing human coronaviruses responses to SARS-CoV-2 immunity. Nat. Commun. 2021, 12, 6703. [Google Scholar] [CrossRef]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Contant, P.; Embong, A.K.; Kanagaiah, P.; Chaves, F.A.; Yang, H.; Branche, A.R.; Topham, D.J.; Sangster, M.Y. S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit. mBio 2020, 11, e01991-20. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Ribes, M.; Vidal, M.; Rubio, R.; Aguilar, R.; Williams, S.; Barrios, D.; Alonso, S.; Hernández-Luis, P.; Mitchell, R.A.; et al. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat. Commun. 2021, 12, 4740. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; De Pauw, I.; Stessel, B.; Dubois, J. Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation. BMC Infect. Dis. 2022, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Pourajam, S.; Kalantari, E.; Talebzadeh, H.; Mellali, H.; Sami, R.; Soltaninejad, F.; Amra, B.; Sajadi, M.; Alenaseri, M.; Kalantari, F.; et al. Secondary Bacterial Infection and Clinical Characteristics in Patients With COVID-19 Admitted to Two Intensive Care Units of an Academic Hospital in Iran During the First Wave of the Pandemic. Front. Cell Infect. Microbiol. 2022, 12, 784130. [Google Scholar] [CrossRef]
- Baindara, P.; Chakraborty, R.; Holliday, Z.M.; Mandal, S.M.; Schrum, A.G. Oral probiotics in coronavirus disease 2019: Connecting the gut-lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes New Infect. 2021, 40, 100837. [Google Scholar] [CrossRef]
- Baindara, P.; Sarker, B.; Earhart, A.P.; Mandal, S.M.; Schrum, A.G. NOTCH signaling in COVID-19: A central hub controlling genes, proteins, and cells that mediate SARS-CoV-2 entry, the inflammatory response, and lung regeneration. Front. Cell Infect. Microbiol. 2022, 12, 928704. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Long, C.; Szczepanski, N.; Griffin, C.; Fitzgerald, A.; Chapin, K. Heterogeneous Longitudinal Antibody Responses to Covid-19 mRNA Vaccination. Clin. Pathol. 2021, 14, 2632010X211049255. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Read, A.F.; Baigent, S.J.; Powers, C.; Kgosana, L.B.; Blackwell, L.; Smith, L.P.; Kennedy, D.; Walkden-Brown, S.W.; Nair, V. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLoS Biol. 2015, 13, e1002198. [Google Scholar] [CrossRef] [Green Version]
- Gandon, S.; Mackinnon, M.J.; Nee, S.; Read, A.F. Imperfect vaccines and the evolution of pathogen virulence. Nature 2001, 414, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef]
- Covid-19 Breakthrough Data. Department of Health. Available online: https://coronavirus.health.ny.gov/covid-19-breakthrough-data (accessed on 6 April 2022).
- Riemersma, K.K.; Grogan, B.E.; Kita-Yarbro, A.; Minor, N.; Eickhoff, J.; Grogan, B.E.; Kita-Yarbro, A.; Halfmann, P.J.; Segaloff, H.E.; Kocharian, A.; et al. Shedding of Infectious SARS-CoV-2 Despite Vaccination. PLoS Pathog. 2021, 18, e1010876, Preprint. [Google Scholar] [CrossRef]
- Acharya, C.B.; Schrom, J.; Mitchell, A.M.; Coil, D.A.; Marquez, C.; Rojas, S.; Wang, C.Y.; Liu, J.; Pilarowski, G.; Solis, L.; et al. No Significant Difference in Viral Load Between Vaccinated and Unvaccinated, Asymptomatic and Symptomatic Groups When Infected with SARS-CoV-2 Delta Variant. MedRxiv 2021. Preprint. [Google Scholar]
- Servellita, V.; Morris, M.K.; Sotomayor-Gonzalez, A.; Gliwa, A.S.; Torres, E.; Brazer, N.; Zhou, A.; Hernandez, K.T.; Sankaran, M.; Wang, B.; et al. Predominance of antibody-resistant SARS-CoV-2 variants in vaccine breakthrough cases from the San Francisco Bay Area, California. Nat. Microbiol. 2022, 7, 277–288. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.-D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Kampf, G. The epidemiological relevance of the COVID-19-vaccinated population is increasing. Lancet 2021, 11. [Google Scholar] [CrossRef]
- Puhach, O.; Adea, K.; Hulo, N.; Sattonnet, P.; Genecand, C.; Iten, A.; Jacquérioz, F.; Kaiser, L.; Vetter, P.; Eckerle, I.; et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022, 28, 1491–1500. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Iyanger, N.; Williams, S.V. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro. Surveill. 2021, 26, 2100092. [Google Scholar] [CrossRef]
- Lan, F.Y.; Sidossis, A.; Iliaki, E.; Buley, J.; Nathan, N.; Bruno-Murtha, L.A.; Kales, S.N. Continued Effectiveness of COVID-19 Vaccination among Urban Healthcare Workers during Delta Variant Predominance. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Huang, J.; Alumkal, J.J.; Zhang, L.; Feng, F.Y.; Thomas, G.V.; Weinstein, A.S.; Friedl, V.; Zhang, C.; Witte, O.N.; et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.; Simmons, R.; Wellington, E.; Cole, M.; Saei, A.; Oguti, B. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: June to November 2020. medRxiv 2021. [Google Scholar] [CrossRef]
- Vitale, J.; Mumoli, N.; Clerici, P.; De Paschale, M.; Evangelista, I.; Cei, M.; Mazzone, A. Assessment of SARS-CoV-2 Reinfection 1 Year After Primary Infection in a Population in Lombardy, Italy. JAMA Intern. Med. 2021, 181, 1407–1408. [Google Scholar] [CrossRef]
- E Flacco, M.; Martellucci, C.A.; Soldato, G.; Carota, R.; Fazii, P.; Caponetti, A.; Manzoli, L. Rate of reinfections after SARS-COV-2 primary infection in the population of an Italian province: A cohort study. J. Public Health 2021. [Google Scholar] [CrossRef]
- Leidi, A.; Koegler, F.; Dumont, R.; Dubos, R.; Zaballa, M.-E.; Piumatti, G.; Coen, M.; Berner, A.; Farhoumand, P.D.; Vetter, P.; et al. Risk of Reinfection After Seroconversion to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Population-based Propensity-score Matched Cohort Study. Clin. Infect. Dis. 2022, 74, 622–629. [Google Scholar] [CrossRef]
- Krutikov, M.; Palmer, T.; Tut, G.; Fuller, C.; Shrotri, M.; Williams, H.; Davies, D.; Irwin-Singer, A.; Robson, J.; Hayward, A.; et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): A prospective cohort study. Lancet Healthy Longev. 2021, 2, e362–e370. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; de Góis Filho, P.G.; Silva, A.M.F.; Santos, J.V.G.; Santos, D.S.; Aquino, M.M.; de Jesus, R.M.; Almeida, M.L.D.; da Silva, J.S.; Altmann, D.M.; et al. Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J. Infect. 2021, 82, 399–406. [Google Scholar] [CrossRef]
- Letizia, A.G.; Ge, Y.; Vangeti, S.; Goforth, C.; Weir, D.L.; A Kuzmina, N.; A Balinsky, C.; Chen, H.W.; Ewing, D.; Soares-Schanoski, A.; et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: A prospective cohort study. Lancet Respir. Med. 2021, 9, 712–720. [Google Scholar] [CrossRef]
- Kojima, N.; Klausner, J.D. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect. Dis. 2022, 22, 12–14. [Google Scholar] [CrossRef]
- Boyton, R.J.; Altmann, D.M. Risk of SARS-COV-2 reinfection after natural infection. Lancet 2021, 397, 1161–1163. [Google Scholar] [CrossRef]
- NY Gov. 2022. Available online: https://coronavirus.health.ny.gov/covid-19-reinfection-data (accessed on 1 February 2022).
- Syed, M.A.; Alnuaimi, A.S.; Qotba, H.A. SARS-CoV-2 seropositivity and subsequent infection risk: A prospective cohort study. IJID Reg. 2022, 2, 21–23. [Google Scholar] [CrossRef]
- Mishra, B.K.; Bhattacharya, D.; Kshatri, J.S. Natural immunity against COVID-19 significantly reduces the risk of reinfection: Findings from a cohort of sero-survey participants. medRxiv 2021. [Google Scholar] [CrossRef]
- Dwyer, C.J.; Cloud, C.A.; Wang, C.; Heidt, P.; Chakraborty, P.; Duke, T.F.; McGue, S.; Jeffcoat, B.; Dunne, J.; Johnson, L.; et al. Comparative analysis of antibodies to SARS-CoV-2 between asymptomatic and convalescent patients. Science 2021, 24, 102489. [Google Scholar] [CrossRef]
- Abu-Raddad, L.; Chemaitelly, H.; Coyle, P. SARS-CoV-2 reinfection in a cohort of 43,000 antibody positive individuals followed for up to 35 weeks. medRxiv 2021. [Google Scholar] [CrossRef]
- Crawford, N.W. Importance of understanding the reinfection risk of COVID-19 in children. Lancet Child Adolesc. Health 2022, 6, 216. [Google Scholar] [CrossRef]
- Hansen, C.H.; Michlmayr, D.; Gubbels, S.M. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population level observational study. Lancet 2021, 397, 1204–1212. [Google Scholar] [CrossRef]
- Perez, G.; Banon, T.; Gazit, S. A 1 to 1000 SARS-CoV-2 reinfection proportion in members of a large healthcare provider in Israel: A preliminary report. medRxiv 2021. [Google Scholar] [CrossRef]
- Pilz, S.; Chakeri, A.; Ioannidis, J.P.; Richter, L.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Allerberger, F. SARS-CoV-2 re-infection risk in Austria. Eur. J. Clin. Investig. 2021, 51, e13520. [Google Scholar] [CrossRef]
- I Qureshi, A.; I Baskett, W.; Huang, W.; Lobanova, I.; Naqvi, S.H.; Shyu, C.-R. Reinfection With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Patients Undergoing Serial Laboratory Testing. Clin. Infect. Dis. 2022, 74, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, M.M.; Reddy, A.J.; Rothberg, M.B. Reinfection Rates Among Patients Who Previously Tested Positive for Coronavirus Disease 2019: A Retrospective Cohort Study. Clin. Infect. Dis. 2021, 73, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef] [PubMed]
- ISS. COVID-19: Sorveglianza, Impatto Delle Infezioni ed Efficacia Vaccinale. Aggiornamento Nazionale 09/02/2022. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_9-febbraio-2022.pdf (accessed on 9 February 2022).
- ISS. COVID-19: Sorveglianza, Impatto Delle Infezioni ed Efficacia Vaccinale. Aggiornamento Nazionale 06/04/2022. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_6-aprile-2022.pdf (accessed on 6 April 2022).
- Murchu, O.E.; Byrne, P.; Carty, P.G.; De Gascun, C.; Keogan, M.; O’Neill, M.; Harrington, P.; Ryan, M. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev. Med. Virol. 2022, 32, e2260. [Google Scholar] [CrossRef]
- Poukka, E.; Baum, U.; Palmu, A.A.; Lehtonen, T.O.; Salo, H.; Nohynek, H.; Leino, T. Cohort study of Covid-19 vaccine effectiveness among healthcare workers in Finland, December 2020–October 2021. Vaccine 2022, 40, 701–705. [Google Scholar] [CrossRef]
- Kojima, N.; Shrestha, N.K.; Klausner, J.D. A Systematic Review of the Protective Effect of Prior SARS-CoV-2 Infection on Repeat Infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Shenai, M.B.; Rahme, R.; Noorchashm, H. Equivalency of Protection from Natural Immunity in COVID-19 Recovered Versus Fully Vaccinated Persons: A Systematic Review and Pooled Analysis. Cureus 2021, 28, e19102. [Google Scholar] [CrossRef]
- Kojima, N.; Roshani, A.; Brobeck, M.; Baca, A.; Klausner, J. Incidence of SARS-CoV-2 infection among previously infected or vaccinated employees. Int. J. Infect. Dis. 2022, 118, 21–23. [Google Scholar] [CrossRef]
- Vaccines and Related Biological Products Advisory Committee Meeting, 10 December 2020; FDA Briefing Document; Pfizer-BioNTech COVID-19 Vaccine. 2020. Available online: https://www.fda.gov/media/144245/download (accessed on 13 September 2021).
- Vaccines and Related Biological Products Advisory Committee Meeting, 17 December 2020; FDA Briefing Document; Moderna COVID-19 Vaccine. 2020. Available online: https://www.fda.gov/media/144434/download (accessed on 13 September 2021).
- Vaccines and Related Biological Products Advisory Committee Meeting, 26 February 2021 FDA Briefing Document; Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. 2021. Available online: https://www.fda.gov/media/146217/download (accessed on 13 September 2021).
- Goldberg, Y.; Mandel, M.; Woodbridge, Y.; Fluss, R.; Novikov, I.; Yaari, R.; Ziv, A.; Freedman, L.; Huppert, A. Similarity of Protection Conferred by Previous SARS-CoV-2 Infection and by BNT162b2 Vaccine: A 3-Month Nationwide Experience from Israel. Am. J. Epidemiol. 2022, 191, 1420–1428. [Google Scholar] [CrossRef]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. SARS-CoV-2 Naturally Acquired Immunity vs. Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, ciac262. [Google Scholar] [CrossRef]
- Satwik, R.; Satwik, A.; Katoch, S.; Saluja, S. ChAdOx1 nCoV-19 effectiveness during an unprecedented surge in SARS COV-2 infections. Eur. J. Intern. Med. 2021, 93, 112–113. [Google Scholar] [CrossRef]
- Wadman, M. Having SARS-CoV-2 once confers much greater immunity than a vaccine—But vaccination remains vital. Science 2021. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2022, 10, 64. [Google Scholar] [CrossRef]
- Sarraf, T.R.; Maity, S.; Ghosh, A.; Bhattacharjee, S.; Pani, A.; Saha, K.; Chattopadhyay, D.; Ghosh, G.; Sen, G.G. Immunity to COVID-19 in India through vaccination and natural infection. MedRix 2021. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef]
- León, T.M.; Dorabawila, V.; Nelson, L.; Lutterloh, E.; Bauer, U.E.; Backenson, B.; Bassett, M.T.; Henry, H.; Bregman, B.; Midgley, C.M.; et al. COVID-19 Cases and Hospitalizations by COVID-19 Vaccination Status and Previous COVID-19 Diagnosis—California and New York, May–November 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Zeng, C.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S.-L. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 2022, 14, eabn8057. [Google Scholar] [CrossRef] [PubMed]
- Eyran, T.; Vaisman-Mentesh, A.; Taussig, D.; Dror, Y.; Aizik, L.; Kigel, A.; Rosenstein, S.; Bahar, Y.; Ini, D.; Tur-Kaspa, R.; et al. The longitudinal kinetics of antibodies in COVID-19 recovered patients over 14 months. MedRxiv 2021. [Google Scholar] [CrossRef]
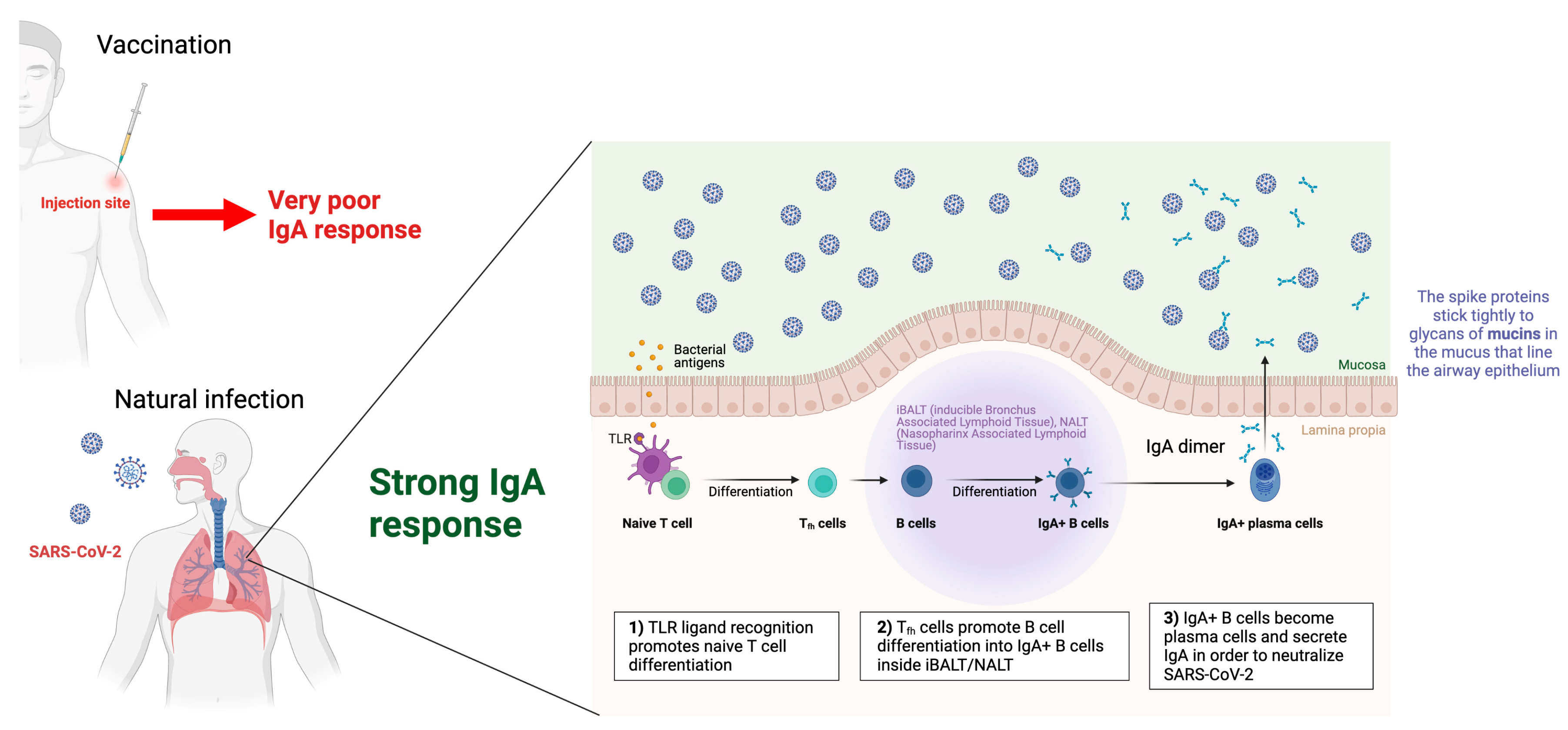
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. eBioMedicine 2022, 75, 103788. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Kim, Y.; Lee, K.-M.; Jang, E.-J.; Woo, C.-H.; Hong, C.-U.; Choi, S.-T.; Xayaheuang, S.; Jang, J.-G.; Ahn, J.-H.; et al. Humoral and Cellular Responses to COVID-19 Vaccines in SARS-CoV-2 Infection-Naïve and -Recovered Korean Individuals. Vaccines 2022, 18, 332. [Google Scholar] [CrossRef]
- Dehgani-Mobaraki, P.; Wang, C.; Floridi, A. Long-Term Persistence of IgG Antibodies in recovered COVID-19 individuals at 18 months and the impact of two-dose BNT162b2 (Pfizer-BioNTech) mRNA vaccination on the antibody response. MedRxiv 2022. [Google Scholar] [CrossRef]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D. Anti- SARS-CoV-2 Receptor Binding Domain Antibody Evolution after MRNA Vaccination. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lyski, Z.L.; E Brunton, A.; I Strnad, M.; E Sullivan, P.; Siegel, S.A.R.; Tafesse, F.G.; Slifka, M.K.; Messer, W.B. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Specific Memory B Cells from Individuals With Diverse Disease Severities Recognize SARS-CoV-2 Variants of Concern. J. Infect. Dis. 2022, 225, 947–956. [Google Scholar] [CrossRef]
- Stamatatos, L.; Czartoski, J.; Wan, Y.-H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Am. Assoc. Adv. Sci. 2021, 372, 1413–1418. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Azman, A.S.; Sun, R.; Lu, W.; Zheng, N.; Zhou, J.; Wu, Q.; Deng, X.; Zhao, Z.; et al. Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants Induced by Natural Infection or Vaccination: A Systematic Review and Pooled Analysis. Clin. Infect. Dis. 2022, 74, 734–742. [Google Scholar] [CrossRef]
- Neidleman, J.; Luo, X.; McGregor, M. mRNA vaccine-induced T cells respond identically to SARS-CoV-2 variants of concern but differ in longevity and homing properties depending on prior infection status. bioRxiv 2021. [Google Scholar] [CrossRef]
- Andeweg, S.P.; Vennema, H.; Veldhuijzen, I.; Smorenburg, N.; Schmitz, D.; Zwagemaker, F.; van Gageldonk-Lafeber, A.B.; Hahné, S.J.M.; Reusken, C.; Knol, M.J.; et al. Elevated risk of infection with SARS-CoV-2 Beta, Gamma, and Delta variant compared to Alpha variant in vaccinated individuals. Sci. Transl. Med. 2022, eabn4338. [Google Scholar] [CrossRef]
- Eythorsson, E.; Runolfsdottir, H.L.; Ingvarsson, R.F.; Sigurdsson, M.I.; Palsson, R. Rate of SARS-CoV-2 Reinfection During an Omicron Wave in Iceland. JAMA Netw. Open 2022, 5, e2225320. [Google Scholar] [CrossRef]
- Sasikala, M.; Shashidhar, J.; Deepika, G.; Ravikanth, V.; Krishna, V.V.; Sadhana, Y.; Pragathi, K.; Reddy, D.N. Immunological memory and neutralizing activity to a single dose of COVID-19 vaccine in previously infected individuals. Int. J. Infect. Dis. 2021, 108, 183–186. [Google Scholar] [CrossRef]
- Callegaro, A.; Borleri, D.; Farina, C.; Napolitano, G.; Valenti, D.; Rizzi, M.; Maggiolo, F. Antibody response to SARS-CoV-2 vaccination is extremely vivacious in subjects with previous SARS-CoV-2 infection. J. Med. Virol. 2021, 93, 4612–4615. [Google Scholar] [CrossRef]
- Anichini, G.; Terrosi, C.; Gandolfo, C.; Savellini, G.G.; Fabrizi, S.; Miceli, G.B.; Cusi, M.G. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N. Engl. J. Med. 2021, 385, 90–92. [Google Scholar] [CrossRef]
- Van Gils, M.J.; van Willigen, H.D.; Wynberg, E.; Han, A.X.; van der Straten, K.; Burger, J.A.; Poniman, M.; Oomen, M.; Tejjani, K.; Bouhuijs, J.H.; et al. A single mRNA vaccine dose in COVID-19 patients boosts neutralizing antibodies against SARS-CoV-2 and variants of concern. Cell Rep. Med. 2021, 14, 100486. [Google Scholar] [CrossRef]
- Lozano-Rodríguez, R.; Valentín-Quiroga, J.; Avendaño-Ortiz, J.; Martín-Quirós, A.; Pascual-Iglesias, A.; Terrón-Arcos, V.; Montalbán-Hernández, K.; Casalvilla-Dueñas, J.C.; Bergón-Gutiérrez, M.; Alcamí, J.; et al. Cellular and humoral functional responses after BNT162b2 mRNA vaccination differ longitudinally between naive and subjects recovered from COVID-19. Cell Rep. 2022, 11, 110235. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Clin. Trial Viruses 2021, 5, 422. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Pajon, R.; Paila, Y.D.; Girard, B.; Dixon, G.; Kacena, K.; Baden, L.R.; El Sahly, H.M.; Essink, B.; Mullane, K.M.; Frank, I.; et al. Initial analysis of viral dynamics and circulating viral variants during the mRNA-1273 Phase 3 COVE trial. Nat. Med. 2022, 28, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Prunas, O.; Warren, J.L.; Crawford, F.W. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Röltgen, K.; Nielsen, S.C.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef] [PubMed]
- Ibarrondo, F.J.; Hofmann, C.; Ali, A.; Ayoub, P.; Kohn, D.B.; Yang, O.O. Previous Infection Combined with Vaccination Produces Neutralizing Antibodies with Potency against SARS-CoV-2 Variants. mBio 2021, 12, e0265621. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Yu, Y.; Esposito, D.; Kang, Z.; Lu, J.; Remaley, A.T.; De Giorgi, V.; Chen, L.N.; West, K.; Cao, L. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci. Rep. 2022, 12, 2628. [Google Scholar] [CrossRef]
- Public Health European Commission. Union Register of Medical Products: Product information. 2022. Available online: https://ec.europa.eu/health/documents/community-register/html/ (accessed on 7 April 2022).
- Ronchini, C.; Gandini, S.; Pasqualato, S.; Mazzarella, L.; Facciotti, F.; Mapelli, M.; Frige’, G.; Passerini, R.; Pase, L.; Capizzi, S.; et al. Lower probability and shorter duration of infections after COVID-19 vaccine correlate with anti-SARS-CoV-2 circulating IgGs. PLoS ONE 2022, 17, e0263014. [Google Scholar] [CrossRef]
- Hansen, C.H.; Blicher, A.; Shelde, A.B.; Moustsen-Helms, I.R.; Emborg, H.D.; Krause, T.G.; Mølbak, K.; Valentiner-Branth, P. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study. MedRxiv 2021. Preprint. [Google Scholar]
- Hammerman, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Peretz, A.; Netzer, D.; Yaron, S.; Arbel, R. Effectiveness of the BNT162b2 Vaccine after Recovery from Covid-19. N. Engl. J. Med. 2022, 386, 1221–1229. [Google Scholar] [CrossRef]
- Gazit, S.; Shlezinger, B.R.; Perez, M.G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, M.E.; Alapi, B.H.; Cohen, D.; Muhsen, K.; et al. The Incidence of SARS-CoV-2 Reinfection in Persons with Naturally Acquired Immunity With and Without Subsequent Receipt of a Single Dose of BNT162b2 Vaccine: A Retrospective Cohort Study. Ann. Intern. Med. 2022, M21-4130. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Coyle, P.; Malek, J.A.; Ahmed, A.A.; Mohamoud, Y.A.; Younuskunju, S.; Ayoub, H.H.; Al Kanaani, Z.; Al Kuwari, E.; et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine 2021, 35, 100861. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Camara, C.; Lopez-Granados, E.; Nozal, P.; del Pino-Molina, L.; Bravo-Gallego, L.Y.; Paz-Artal, E.; Pion, M.; Correa-Rocha, R.; Ortiz, A.; et al. Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naive and COVID-19 recovered individuals. Cell Rep. 2021, 24, 109570. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, 58. [Google Scholar] [CrossRef]
- Yu, J.; Collier, A.; Rowe, M. Comparable Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants. Medrxiv 2022. Preprint. [Google Scholar] [CrossRef]
- Ledford, H. ‘Killer’ immune cells still recognize Omicron variant. Nature 2022, 601, 307. [Google Scholar] [CrossRef]
- Flemming, A. Omicron, the great escape artist. Nat. Rev. Immunol. 2022, 22, 75. [Google Scholar] [CrossRef]
- Araf, Y.; Akter, F.; Tang, Y.; Fatemi, R.; Alam Parvez, S.; Zheng, C.; Hossain, G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- Bansal, K.; Kumar, S. Mutational cascade of SARS-CoV-2 leading to evolution and emergence of omicron variant. bioRxiv 2021, 2012, 471389. [Google Scholar] [CrossRef]
- Kozlov, M. Omicron’s feeble attack on the lungs could make it less dangerous. Nature 2022, 601, 177. [Google Scholar] [CrossRef]
- Bager, P.; Wohlfahrt, J.; Bhatt, S.; Stegger, M.; Legarth, R.; Møller, C.H.; Skov, R.L.; Valentiner-Branth, P.; Voldstedlund, M.; Fischer, T.K.; et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: An observational cohort study. Lancet Infect. Dis. 2022, 22, 967–976. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Jung, C.; Kmiec, D.; Koepke, L.; Zech, F.; Jacob, T.; Sparrer, K.M.J.; Kirchhoff, F. Omicron: What Makes the Latest SARS-CoV-2 Variant of Concern So Concerning? J. Virol. 2022, 96, e0207721. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.; Ghani, A.; Hinsley, W. Report 50: Hospitalisation risk for Omicron cases in England. Imp. Coll. Lond. 2021. [Google Scholar] [CrossRef]
- ISS. Bollettino 18 Febbraio 2022, Variante Omicron Dominante. Available online: https://www.iss.it/web/guest/cov19-cosa-fa-iss-varianti/-/asset_publisher/yJS4xO2fauqM/content/flash-survey-31-gennaio-2022-variante-omicron-al-99-?_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_assetEntryId=6697267&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_redirect=https://www.iss.it/web/guest/cov19-cosa-fa-iss-varianti?p_p_id=com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_assetEntryId=6697267&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_cur=0&p_r_p_resetCur=false (accessed on 18 February 2022).
- Wang, L.; Berger, N.A.; Kaelber, D.C.; Davis, P.B.; Volkow, N.D.; Xu, R. Incidence Rates and Clinical Outcomes of SARS-CoV-2 Infection with the Omicron and Delta Variants in Children Younger Than 5 Years in the US. JAMA Pediatr. 2022, 176, 811–813. [Google Scholar] [CrossRef]
- Martellucci, C.A.; Flacco, M.E.; Soldato, G.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Effectiveness of COVID-19 Vaccines in the General Population of an Italian Region before and during the Omicron Wave. Vaccines 2022, 10, 662. [Google Scholar] [CrossRef]
- Xia, S.; Wang, L.; Zhu, Y.; Lu, L.; Jiang, S. Origin, virological features, immune evasion and intervention of SARS-CoV-2 Omicron sublineages. Signal Transduct. Target Ther. 2022, 7, 241. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradnik, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H. Protection of SARS-CoV-2 natural infection against reinfection with the Omicron BA.4 or BA.5 subvariants. medRxiv 2022. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N. Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar] [CrossRef]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet 2022, 399, 437–444. [Google Scholar] [CrossRef]
- Fohse, F.K.; Geckin, B.; Overheul, G.J. The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses. MedRxiv 2021. Preprint. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, C.; Grifoni, A.; Müller, T.R.; Niessl, J.; Olofsson, A.; Humbert, M.; Hansson, L.; Österborg, A.; Bergman, P.; et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 2022, 28, 472–476. [Google Scholar] [CrossRef]
- Ledford, H. How severe are Omicron infections? Nature 2021, 600, 577–578. [Google Scholar] [CrossRef]
- Flacco, M.E.; Soldato, G.; Martellucci, C.A.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Risk of SARS-CoV-2 Reinfection 18 Months After Primary Infection: Population-Level Observational Study. Front. Public Health 2022, 10, 884121. [Google Scholar] [CrossRef]
- Gagne, M.; Moliva, J.I.; Foulds, K.E.; Andrew, S.F.; Flynn, B.J.; Werner, A.P.; Wagner, D.A.; Teng, I.-T.; Lin, B.C.; Moore, C.; et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing responses, and protection from Omicron. Cell 2022, 185, 1556–1571.e18. [Google Scholar] [CrossRef]
- WHO. Interim Statement on COVID-19 Vaccines in the Context of the Circulation of the Omicron SARS-CoV-2 Variant from the WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC, 2022). Available online: https://www.who.int/news/item/11-01-2022-interim-statement-on-covid-19-vaccines-in-the-context-of-the-circulation-of-the-omicron-sars-cov-2-variant-from-the-who-technical-advisory-group-on-covid-19-vaccine-composition (accessed on 11 January 2022).
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; A Burgers, W.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- Elliott, P.; Eales, O.; Steyn, N.; Tang, D.; Bodinier, B.; Wang, H.; Elliott, J.; Whitaker, M.; Atchison, C.; Diggle, P.J.; et al. Post-peak dynamics of a national Omicron SARS-CoV-2 epidemic during January 2022. Science 2022, eabq4411. [Google Scholar] [CrossRef]
- Boucau, J.; Marino, C.; Regan, J.; Uddin, R.; Choudhary, M.C.; Flynn, J.P.; Chen, G.; Stuckwisch, A.M.; Mathews, J.; Liew, M.Y.; et al. Duration of Shedding of Culturable Virus in SARS-CoV-2 Omicron (BA.1) Infection. N. Engl. J. Med. 2022, 387, 275–277. [Google Scholar] [CrossRef]
- Ying, B.; Scheaffer, S.M.; Whitener, B. Boosting with Omicron-matched or historical mRNA vaccines increases neutralizing antibody responses and protection against B.1.1.529 infection in mice. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hawman, D.W.; Meade-White, K.; Clancy, C.; Archer, J.; Hinkley, T.; Leventhal, S.S.; Rao, D.; Stamper, A.; Lewis, M.; Rosenke, R.; et al. Replicating RNA platform enables rapid response to the SARS-CoV-2 Omicron variant and elicits enhanced protection in naïve hamsters compared to ancestral vaccine. EBioMedicine 2022, 83, 104196. [Google Scholar] [CrossRef] [PubMed]
- Windsor, I.W.; Tong, P.; Lavidor, O.; Moghaddam, A.S.; McKay, L.G.; Gautam, A.; Chen, Y.; MacDonald, E.A.; Yoo, D.K.; Griffths, A.; et al. Antibodies induced by an ancestral SARS-CoV-2 strain that cross-neutralize variants from Alpha to Omicron BA.1. Sci. Immunol. 2022, 7, eabo3425. [Google Scholar] [CrossRef] [PubMed]
- Tsumiyama, K.; Miyazaki, Y.; Shiozawa, S. Self-organized criticality theory of autoimmunity. PLoS ONE 2009, 4, e8382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, R.; Azzolini, E.; Pozzi, C.; Ubaldi, L.; Lagioia, M.; Mantovani, A.; Rescigno, M. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in individuals who have recovered from symptomatic COVID-19. J. Clin. Investig. 2021, 131, e149154. [Google Scholar] [CrossRef]
- Debes, A.K.; Xiao, S.; Colantuoni, E.; Egbert, E.R.; Caturegli, P.; Gadala, A.; Milstone, A.M. Association of Vaccine Type and Prior SARS-CoV-2 Infection with Symptoms and Antibody Measurements Following Vaccination Among Health Care Workers. JAMA Intern. Med. 2021, 181, 1660–1662. [Google Scholar] [CrossRef]
- Raw, R.K.; Kelly, C.A.; Rees, J.; Wroe, C.; Chadwick, D.R. Previous COVID-19 infection, but not Long-COVID, is associated with increased adverse events following BNT162b2/Pfizer vaccination. J. Infect. 2021, 83, 381–412. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Expected Viscosity After COVID-19 Vaccination, Hyperviscosity and Previous COVID-19. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211020833. [Google Scholar] [CrossRef]
- Zappa, M.; Verdecchia, P.; Spanevello, A.; Visca, D.; Angeli, F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur. J. Intern. Med. 2021, 90, 111–113. [Google Scholar] [CrossRef]
- Talotta, R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2021, 224, 108665. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Ghrew, M.; Ustianowski, A.; Ahmad, S.; Borrow, R.; Papavasileiou, L.P.; Petrakis, D.; Bakerly, N.D. Self-reported real-world safety and reactogenicity of COVID-19 vaccines: An international vaccine-recipient survey. Life 2021, 11, 249. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Cupaiolo, R.; Papleux, E.; Wilmet, A.; Horeanga, A.; Antoine-Moussiaux, T.; Della Vecchia, A.; Beukinga, I.; Vekemans, M.; Blairon, L. Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef]
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021, 36, 427–439. [Google Scholar] [CrossRef]
- Brazete, C.; Aguiar, A.; Furtado, I.; Duarte, R. Thrombotic events and COVID-19 vaccines. Int. J. Tuberc. Lung Dis. 2021, 25, 701–707. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2022, 43, 3–40. [Google Scholar] [CrossRef]
- Elrashdy, F.; Tambuwala, M.M.; Hassan, S.S.; Adadi, P.; Seyran, M.; El-Aziz, T.M.A.; Rezaei, N.; Lal, A.; Aljabali, A.A.; Kandimalla, R.; et al. Autoimmunity roots of the thrombotic events after COVID-19 vaccination. Autoimmun. Rev. 2021, 20, 102941. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Chen, Y.; Williams, A.P.; Gao, Y.; Zeng, J. Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials. Vaccines 2021, 9, 939. [Google Scholar] [CrossRef]
- Lai, C.-C.; Chen, I.-T.; Chao, C.-M.; Lee, P.-I.; Ko, W.-C.; Hsueh, P.-R. COVID-19 vaccines: Concerns beyond protective efficacy and safety. Expert Rev. Vaccines 2021, 20, 1013–1025. [Google Scholar] [CrossRef]
- Sharma, K.; Patel, S.; Patel, Z.; Patel, K.B.; Doshi, J.S.; Shah, D.B.; Chokshi, P.; Parbatani, A.; Sharma, C.; Patel, A.; et al. A Comprehensive Analysis of Myocarditis in Formerly Healthy Individuals Following SARS-CoV-2 Vaccination (COVID-19 Immunization). Cureus 2022, 14, e26851. [Google Scholar] [CrossRef]
- Lee, K.M.N.; Junkins, E.J.; Luo, C.; Fatima, U.A.; Cox, M.L.; Clancy, K.B.H. Investigating trends in those who experience menstrual bleeding changes after SARS-CoV-2 vaccination. Sci. Adv. 2022, 8, eabm7201. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S.; Dardiotis, E. Current Evidence in SARS-CoV-2 mRNA Vaccines and Post-Vaccination Adverse Reports: Knowns and Unknowns. Diagnostics 2022, 12, 1555. [Google Scholar] [CrossRef] [PubMed]
- ElSawi, H.A.; Elborollosy, A. Immune-mediated adverse events post-COVID vaccination and types of vaccines: A systematic review and meta-analysis. Egypt J. Intern. 2022, 34, 44. [Google Scholar] [CrossRef] [PubMed]
- Abas, A.H.; Marfuah, S.; Idroes, R.; Kusumawaty, D.; Fatimawali; Park, M.N.; Siyadatpanah, A.; Alhumaydhi, F.A.; Mahmud, S.; Tallei, T.E.; et al. Can the SARS-CoV-2 Omicron Variant Confer Natural Immunity against COVID-19? Molecules 2022, 27, 2221. [Google Scholar] [CrossRef]
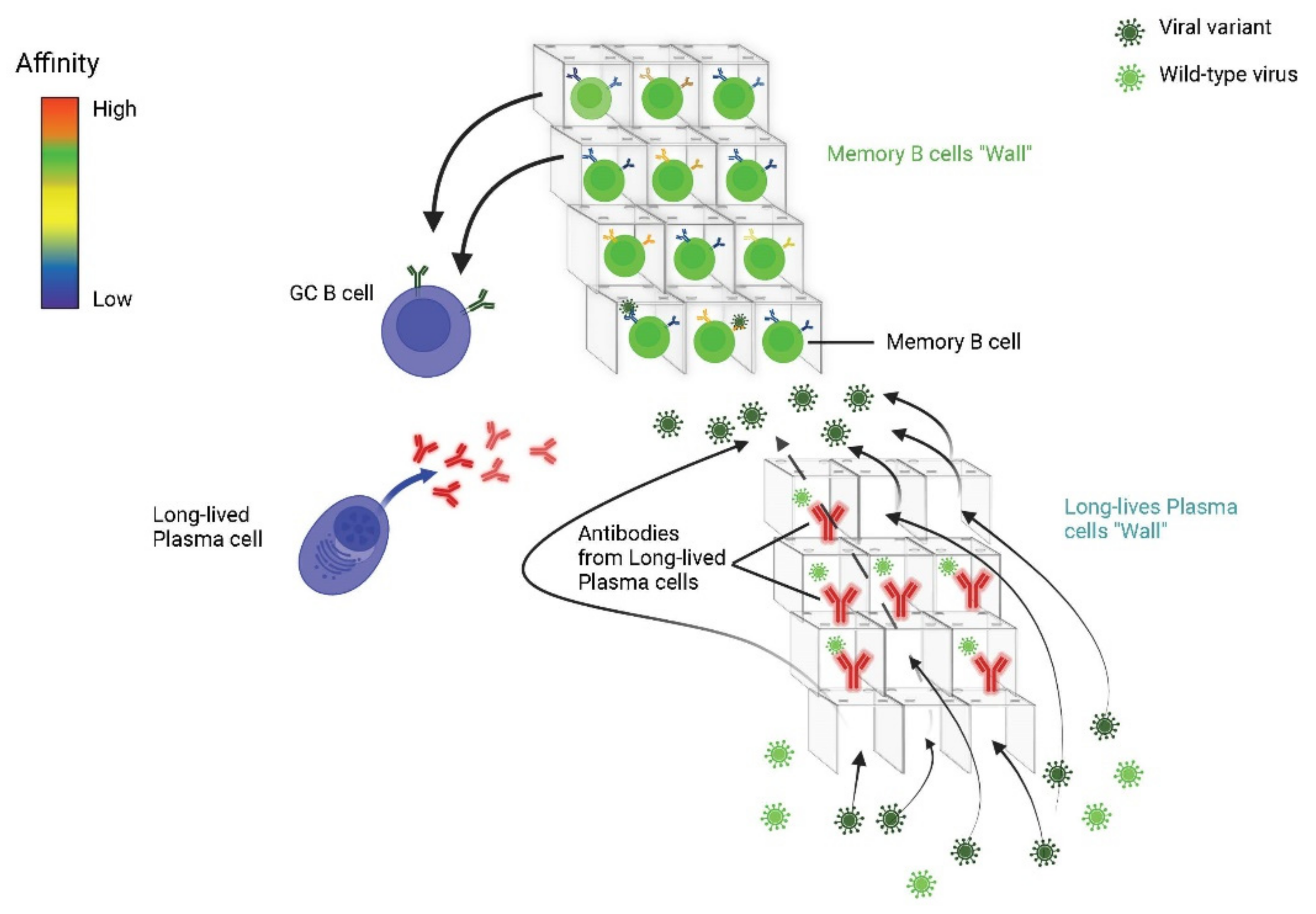
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Tsumiyama, K.; Yamane, T.; Ito, M.; Shiozawa, S. Self-Organized Criticality Theory and the Expansion of PD-1-Positive Effector CD4 T Cells: Search for Autoantibody-Inducing CD4 T Cells. Front. Immunol. 2013, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Shiozawa, S.; Tsumiyama, K.; Miyazaki, Y.; Uto, K.; Sakurai, K.; Nakashima, T.; Matsuyama, H.; Doi, A.; Tarui, M.; Izumikawa, M.; et al. DOCK8-expressing T follicular helper cells newly generated beyond self-organized criticality cause systemic lupus erythematosus. iScience 2021, 25, 103537. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Terpeluk, P.; Gordon, S.M. Necessity of COVID-19 vaccination in previously infected individuals. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.06.01.21258176v2 (accessed on 5 June 2021).
- McGonagle, D.G. Health-care workers recovered from natural SARS-CoV-2 infection should be exempt from mandatory vaccination edicts. Lancet Rheumatol. 2022, 4, e170. [Google Scholar] [CrossRef]


| Study | Population | Follow-Up | Outcomes |
|---|---|---|---|
| Abu-Raddad et al., 2021 [130] | General population N = 43.044 antibody-positive at baseline | Median: 114 days Maximum: 242 days | Risk of reinfection: 0.1% (95% CI 0.08%–0.11%) |
| Crawford NW, 2022 [131] | General population N = 688.418 PCR positive at baseline | 515 days | Risk of reinfection by age: <5 years 0.18% 5–11 years 0.24% 12–16 years 0.49% >16 years 0.73% |
| Dos Santos et al., 2021 [122] | Healthcare workers N = 378 qRT-PCR positive at baseline | Median: 41 days Maximum: 130 days | Risk of COVID-19 recurrence: 7.9% (both reappearance of the same virus and new infections) 1 Virus genome sequencing identified reinfection (0.26%) |
| Flacco et al., 2021 [119] | General population N = 7173 PCR positive at baseline | Median: 201 days Maximum: 414 days | Risk of reinfection: 0.33% Risk of hospitalization: 0.06% Risk of lethal events: 0.01% |
| Hall et al., 2021 [117] | Healthcare workers N = 6.614 antibody-positive at baseline | Median: 202 days Maximum: 227 days | Adjusted odds ratio of probable reinfection: 0.1 (95% CI 0.00–0.03) |
| Hanrath et al., 2020 [12] | Healthcare workers N = 1.038 PCR or antibody-positive at baseline | Median: 173 days Maximum: 229 days | Symptomatic reinfection: 0% (95% CI 0%-0.4%) |
| Hansen et al., 2021 [132] | General population N = 11.068 PCR positive at baseline | Median: 122 days Maximum: 295 days | Relative risk: 0.20 (0.16–0.25) |
| Harvey et al., 2020 [9] | General population N = 378.606 PCR positive at baseline | >90 days after first infection | Risk of reinfection: 0.3% Relative risk: 0.10 (95% CI 0.05–0.19) declining over time |
| Houlihan et al., 2020 [11] | Healthcare workers N = 33 antibody-positive at baseline | 90 days | 1 PCR positive on days 8 and 13 after enrolment (probable reappearance of the same virus) |
| Jeffery-Smith et al., 2021 [114] | Staff & residents at care homes N = 88 PCR or antibody-positive at baseline | 120 days | Risk of reinfection: 1.1% Relative risk: 0.04 (95% CI 0.005–0.27) |
| Krutikov et al., 2021 [121] | Staff & residents at care homes N = 634 antibody-positive at baseline | Median: 79 days Maximum: 300 days | Relative adjusted hazard ratios (any reinfection): Residents of care home: 0.15 (0.05–0.44); Staff of care home: 0.39 (0.19–0.82) |
| Leidi et al., 2022 [120] | General population N = 498 antibody-positive at baseline | Median: 35,6 weeks Maximum: 38,8 weeks | Risk of reinfection: 1% |
| Letizia et al., 2021 [123] | Marines N = 189 | 6 weeks | Risk of reinfection: 10% Relative risk: 0.45 (95% CI 0.32–0.65) |
| Lumley et al., 2021 [116] | Healthcare workers N = 1.265 antibody-positive at baseline | Median: 139 days Maximum: 217 days | Risk of reinfection: 0.16% Relative risk: 0.11 (95% CI 0.03–0.44) |
| Mishra et al., 2021 [128] | General population N = 1170 antibody-positive at baseline | Median: 258 days Maximum: 319 days | Risk of reinfection: 0.26% Relative risk: 0.023 (95% CI: 0.007–0.073) Risk of hospitalization: 0.08% Risk of lethal events: 0% |
| Perez et al., 2021 [133] | General population N = 149.735 PCR positive at baseline | Median: 165 days Maximum: 325 days ca. | Risk of reinfection: 0.1% |
| Pilz et al., 2021 [134] | General population N = 14.840 PCR positive at baseline | Median: 210 days Maximum: 300 days | Risk of reinfection: 0.27% Relative risk: 0.09 (95% CI: 0.07–0.13) |
| Qureshi et al., 2022 [135] | General population N = 9119 positive | Median: 116 days Maximum: 137 days | Risk of reinfection: 0.7% (95%, CI: 0.5%-0.9%) declining over time |
| Sheehan et al., 2021 [136] | General population N = 8.845 PCR positive at baseline | 90 days after first infection | Protective effectiveness (any reinfection): 78.5% (95% CI: 72.0%–83.5% growing over time |
| Vitale et al., 2021 [118] | General population N = 1597 PCR positive at baseline | Median: 280 days Maximum: 321 days | Risk of reinfection: 0.31%; (95% CI, 0.03%-0.58%) Risk of hospitalization: 0.06% Risk of lethal events: 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diani, S.; Leonardi, E.; Cavezzi, A.; Ferrari, S.; Iacono, O.; Limoli, A.; Bouslenko, Z.; Natalini, D.; Conti, S.; Mantovani, M.; et al. SARS-CoV-2—The Role of Natural Immunity: A Narrative Review. J. Clin. Med. 2022, 11, 6272. https://doi.org/10.3390/jcm11216272
Diani S, Leonardi E, Cavezzi A, Ferrari S, Iacono O, Limoli A, Bouslenko Z, Natalini D, Conti S, Mantovani M, et al. SARS-CoV-2—The Role of Natural Immunity: A Narrative Review. Journal of Clinical Medicine. 2022; 11(21):6272. https://doi.org/10.3390/jcm11216272
Chicago/Turabian StyleDiani, Sara, Erika Leonardi, Attilio Cavezzi, Simona Ferrari, Oriana Iacono, Alice Limoli, Zoe Bouslenko, Daniele Natalini, Stefania Conti, Mauro Mantovani, and et al. 2022. "SARS-CoV-2—The Role of Natural Immunity: A Narrative Review" Journal of Clinical Medicine 11, no. 21: 6272. https://doi.org/10.3390/jcm11216272
APA StyleDiani, S., Leonardi, E., Cavezzi, A., Ferrari, S., Iacono, O., Limoli, A., Bouslenko, Z., Natalini, D., Conti, S., Mantovani, M., Tramonte, S., Donzelli, A., & Serravalle, E. (2022). SARS-CoV-2—The Role of Natural Immunity: A Narrative Review. Journal of Clinical Medicine, 11(21), 6272. https://doi.org/10.3390/jcm11216272







