Complications in Spinal Fusion Surgery: A Systematic Review of Clinically Used Cages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
- (1)
- In PubMed: (i) types of papers (Clinical Study; Clinical Trial; Clinical Trial, Phase I; Clinical Trial, Phase II; Clinical Trial, Phase III; Clinical Trial, Phase IV; Comparative Study; Multicenter Study; Randomized Controlled Trial); (ii) language (English); and (iii) publication date (from 1 January 2011 to 1 January 2021),
- (2)
- In Scopus and Web of Science: (i) language (English); (ii) publication date (between 2011 and 2021); and (iii) types of papers (articles).
2.3. Information Extracted from Articles
2.4. Risk of Bias Assessment
3. Results
3.1. Lumbar SF
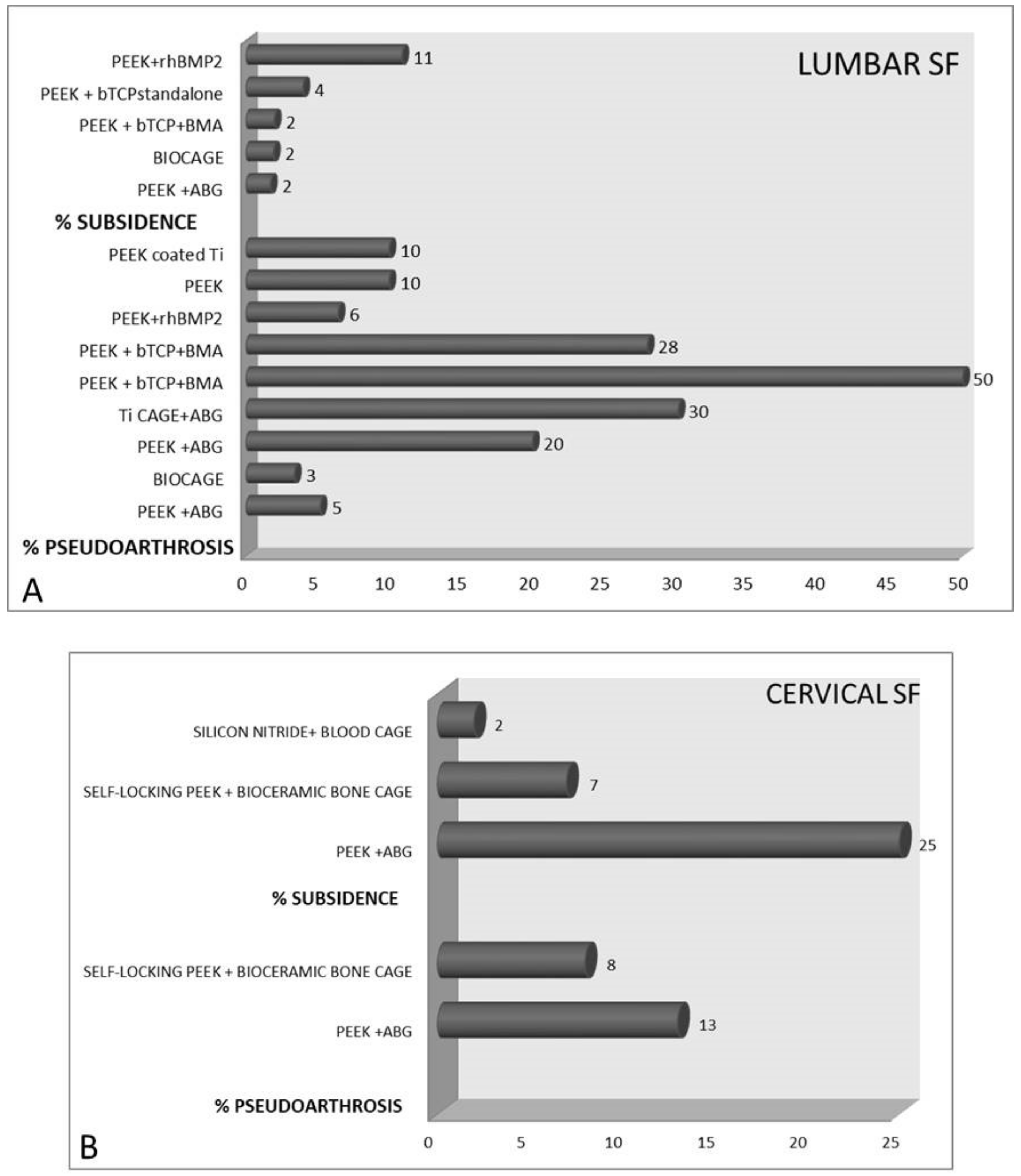
3.2. Complications
3.3. Clinical Outcomes
3.4. Anterior Cervical Discectomy and Fusion
3.5. Complications
3.6. Clinical Outcomes
3.7. Risk of Bias Assessment
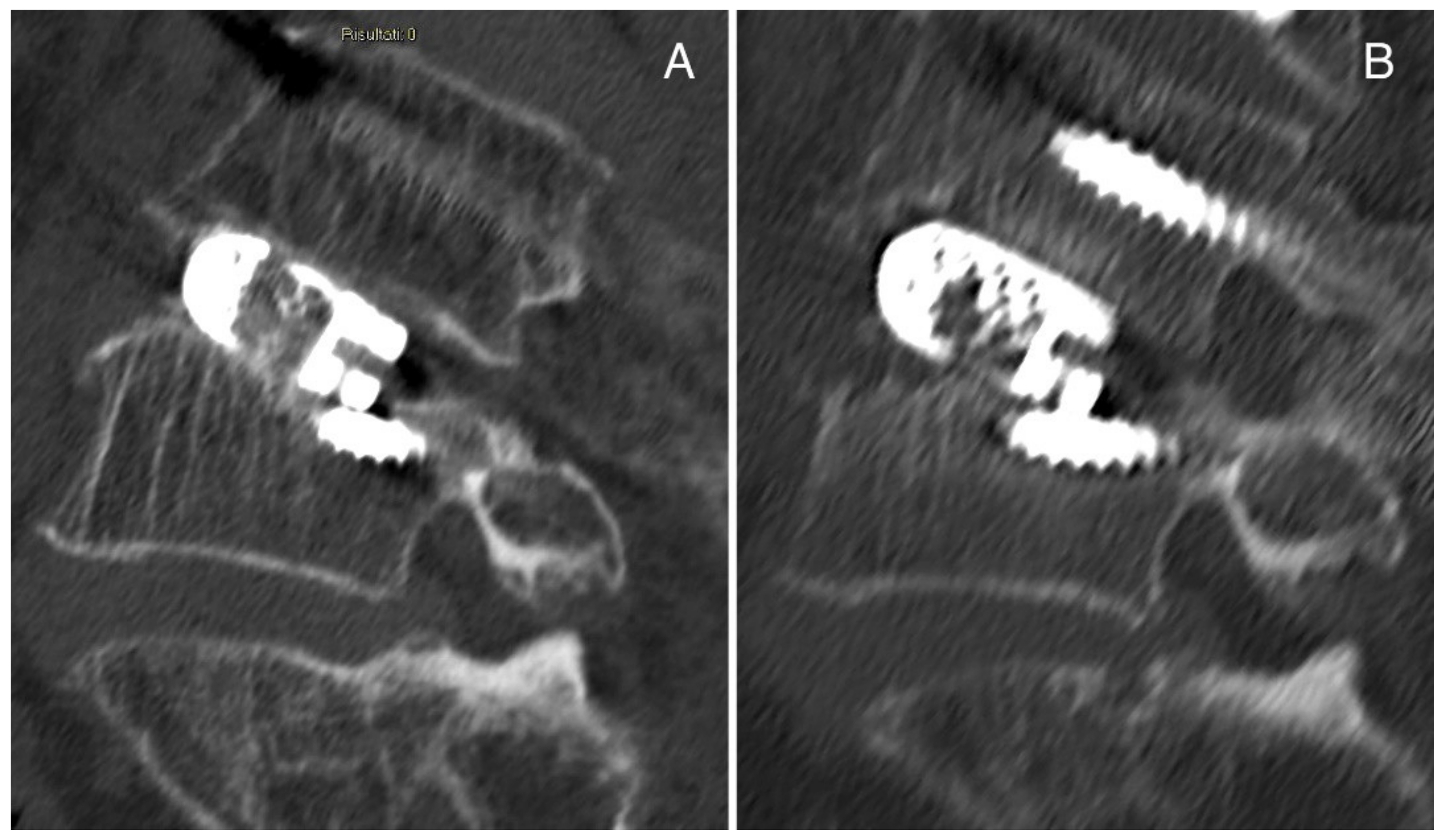
3.8. Representative Cases of Complications from Our Institution
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Kukkar, N.; Sharif, K.; Main, B.J.; Albers, C.E.; El-Amin Iii, S.F. Bone graft substitutes for spine fusion: A brief review. World J. Orthop. 2015, 6, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Buser, Z.; Brodke, D.S.; Youssef, J.A.; Meisel, H.J.; Myhre, S.L.; Hashimoto, R.; Park, J.B.; Yoon, S.T.; Wang, J.C. Synthetic bone graft versus autograft or allograft for spinal fusion: A systematic review. J. Neurosurg. Spine 2016, 25, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.T.; Yang, X.G.; Wang, F.; He, X.; Hu, Y.C. Efficacy and safety of bone substitutes in lumbar spinal fusion: A systematic review and network meta-analysis of randomized controlled trials. Eur. Spine J. 2020, 29, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Tschon, M.; Borsari, V.; Pagani, S.; Martini, L.; Fini, M. Spinal fusion procedures in the adult and young population: A systematic review on allogenic bone and synthetic grafts when compared to autologous bone. J. Mater. Sci. Mater. Med. 2020, 31, 51. [Google Scholar] [CrossRef]
- Girolami, M.; Sartori, M.; Monopoli-Forleo, D.; Ghermandi, R.; Tedesco, G.; Evangelisti, G.; Pipola, V.; Pesce, E.; Falzetti, L.; Fini, M.; et al. Histological examination of a retrieved custom-made 3D-printed titanium vertebra: Do the fine details obtained by additive manufacturing really promote osteointegration? Eur. Spine J. 2021, 30, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- Merriwether, M.; Shockey, R. (Inventors) Box cage for intervertebral body fusion. U.S. Patent 19,990,436,593, 9 November 1999. [Google Scholar]
- Kersten, R.F.; van Gaalen, S.M.; de Gast, A.; Öner, F.C. Polyetherether-ketone (PEEK) cages in cervical applications: A systematic review. Spine J. 2015, 15, 1446–1460. [Google Scholar] [CrossRef]
- Seaman, S.; Kerezoudis, P.; Bydon, M.; Torner, J.C.; Hitchon, P.W. Titanium vs. poly-etheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J. Clin. Neurosci. 2017, 44, 23–29. [Google Scholar] [CrossRef]
- McGilvray, K.C.; Easley, J.; Seim, H.B.; Regan, D.; Berven, S.H.; Hsu, W.K.; Mroz, T.E.; Puttlitz, C.M. Bony ingrowth potential of 3D-printed porous titanium alloy: A direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J. 2018, 18, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Majd, M.E.; Vadhva, M.; Holt, R.T. Anterior cervical reconstruction using titanium cages with anterior plating. Spine 1999, 24, 1604–1610. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement--a materials science perspective. Biomaterials 1999, 19, 1621–1639. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Uri, O.; Folman, Y.; Laufer, G.; Behrbalk, E. A Novel Spine Fixation System Made Entirely of Carbon-Fiber-Reinforced PEEK Composite: An In Vitro Mechanical Evaluation. Adv. Orthop. 2020, 2020, 4796136. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, P.; Chen, Q.; Thu, H.E.; Hussain, Z. Current Updates on Bone Grafting Biomaterials and Recombinant Human Growth Factors Implanted Biotherapy for spinal fusion: A Review of Human Clinical Studies. Curr. Drug Deliv. 2019, 16, 94–110. [Google Scholar] [CrossRef]
- Verla, T.; Xu, D.S.; Davis, M.J.; Reece, E.M.; Kelly, M.; Nunez, M.; Winocour, S.J.; Ropper, A.E. Failure in Cervical spinal fusion and Current Management Modalities. Semin. Plast. Surg. 2021, 35, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Long, X.; Shi, L.; Wang, Y.; Guan, T.; Lv, J.; Cai, L. Prevalence and risk factors for cage subsidence after lumbar interbody fusion: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e28085. [Google Scholar] [CrossRef]
- Kon, E.; Verdonk, P.; Condello, V.; Delcogliano, M.; Dhollander, A.; Filardo, G.; Pignotti, E.; Marcacci, M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: Systematic clinical data review and study quality analysis. Am. J. Sports Med. 2009, 37, 156S–166S. [Google Scholar] [CrossRef]
- Lin, B.; Yu, H.; Chen, Z.; Huang, Z.; Zhang, W. Comparison of the PEEK cage and an autologous cage made from the lumbar spinous process and laminae in posterior lumbar interbody fusion. BMC Musculoskelet. Disord. 2016, 17, 374. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.J.; Li, Y.; Hou, T.Y.; Cheng, P.; Zhang, Z.H.; Xu, J.Z.; Luo, F. Application of New Allogeneic Lumbar Fusion Cage (Biocage) in Single-Segment Lumbar Degenerative Disease: A Prospective Controlled Study with Follow-Up for ≥2 Years. World Neurosurg. 2019, 126, e1309–e1314. [Google Scholar] [CrossRef]
- Thaler, M.; Lechner, R.; Gstottner, M.; Kobel, C.; Bach, C. The use of beta-tricalcium phosphate and bone marrow aspirate as a bone graft substitute in posterior lumbar interbody fusion. Eur. Spine J. 2013, 22, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Tanida, S.; Fujibayashi, S.; Otsuki, B.; Masamoto, K.; Takahashi, Y.; Nakayama, T.; Matsuda, S. Vertebral Endplate Cyst as a Predictor of Nonunion After Lumbar Interbody Fusion Comparison of Titanium and Polyetheretherketone Cages. Spine 2016, 41, E1216–E1222. [Google Scholar] [CrossRef] [PubMed]
- Oikonomidis, S.; Ashqar, G.; Kaulhausen, T.; Herren, C.; Siewe, J.; Sobottke, R. Clinical experiences with a PEEK-based dynamic instrumentation device in lumbar spinal surgery: 2 years and no more. J. Orthop. Surg. Res. 2018, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Rickert, M.; Fleege, C.; Tarhan, T.; Schreiner, S.; Makowski, M.R.; Rauschmann, M.; Arabmotlagh, M. Transforaminal lumbar interbody fusion using polyetheretherketone oblique cages with and without a titanium coating. A Randomised Clin. Pilot Study Bone Jt. J. 2017, 99-B, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Lechner, R.; Putzer, D.; Liebensteiner, M.; Bach, C.; Thaler, M. Fusion rate and clinical outcome in anterior lumbar interbody fusion with beta-tricalcium phosphate and bone marrow aspirate as a bone graft substitute. A prospective clinical study in fifty patients. Int. Orthop. 2017, 41, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Malham, G.M.; Parker, R.M.; Ellis, N.J.; Blecher, C.M.; Chow, F.Y.; Claydon, M.H. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein–2: A prospective study of Complications. Clinical article. J. Neurosurg. Spine 2014, 21, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Parker, R.M.; Malham, G.M. Comparison of a calcium phosphate bone substitute with recombinant human bone morphogenetic protein-2: A prospective study of fusion rates, clinical outcomes and complications with 24-month follow-up. Eur. Spine J. 2017, 26, 754–763. [Google Scholar] [CrossRef]
- Landriel, F.A.; Hem, S.; Goldschmidt, E.; Ajler, P.; Vecchi, E.; Carrizo, A. Polyetheretherketone interbody cages versus autogenous iliac crest bone grafts with anterior fixation for cervical disc disease. J. Spinal Disord. Tech. 2013, 26, 61–67. [Google Scholar] [CrossRef]
- Liu, J.M.; Xiong, X.; Peng, A.F.; Xu, M.; Chen, X.Y.; Long, X.H.; Xu, R.; Liu, Z.L. A comparison of local bone graft with PEEK cage versus iliac bonegraft used in anterior cervical discectomy and fusion. Clin. Neurol. Neurosurg. 2017, 155, 30–35. [Google Scholar] [CrossRef]
- El-Tantawy, A. Is it possible to eliminate the plate-related problems and still achieve satisfactory outcome after multilevel anterior cervical discectomy? Eur. J. Orthop. Surg. Traumatol. 2015, 25, S135–S145. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, G.; Wang, B.; Li, L.; Kuang, L. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking stand-alone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: A retrospective study with 2-year follow-up. Eur. Spine J. 2016, 25, 2255–2262. [Google Scholar]
- Arts, M.P.; Wolfs, J.F.C.; Corbin, T.P. Porous silicon nitride spacers versus PEEK cages for anterior cervical discectomy and fusion: Clinical and radiological results of a single-blinded randomized controlled trial. Eur. Spine J. 2017, 26, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.; Hu, Y.; Lyu, Q.; Liu, L.; Zhu, C.; Zhou, C.; Song, Y. The n-HA/PA66 Cage Versus the PEEK Cage in Anterior Cervical Fusion with Single-Level Discectomy During 7 Years of Follow-Up. World Neurosurg. 2019, 123, e678–e684. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, H.; Yuan, J.; Fu, L.; Yang, J.; Zhang, P. A prospective randomized comparison of PEEK cage containing calcium sulphate or demineralized bone matrix with autograft in anterior cervical interbody fusion. Int. Orthop. 2015, 39, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Roh, S.W. Anterior cervical interbody fusion using polyetheretherketone cage filled with autologous and synthetic bone graft substrates for cervical spondylosis: Comparative analysis between PolyBone and iliac bone. Neurol. Med. Chir. 2013, 53, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Farrokhi, M.R.; Nikoo, Z.; Gholami, M.; Hosseini, K. Comparison Between Acrylic Cage and Polyetheretherketone (PEEK) Cage in Single-level Anterior Cervical Discectomy and Fusion. A Randomized Clinical Trial. Clin. Spine Surg. 2017, 30, 38–46. [Google Scholar] [CrossRef]
- Lovasik, B.P.; Holland, C.M.; Howard, B.M.; Baum, G.R.; Rodts, G.E.; Refai, D. Anterior Cervical Discectomy and Fusion: Comparison of Fusion, Dysphagia, and Complication Rates Between Recombinant Human Bone Morphogenetic Protein-2 and Beta-Tricalcium Phosphate. World Neurosurg. 2017, 97, 674–683. [Google Scholar] [CrossRef]
- Chong, E.; Mobbs, R.J.; Pelletier, M.H.; Walsh, W.R. Titanium/Polyetheretherketone Cages for Cervical Arthrodesis with Degenerative and Traumatic Pathologies: Early Clinical Outcomes and Fusion Rates. Orthop. Surg. 2016, 8, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Yasuda, M.; Niwa, A.; Wakao, N.; Nakura, T.; Osuka, K.; Kamiya, M.; Takayasu, M. Plasmapore-Coated Titanium Cervical Cages Induce More Rapid and Complete Bone Fusion After Anterior Cervical Discectomy and Fusion as Compared to Noncoated Titanium Cages. World Neurosurg. 2014, 82, 519–522. [Google Scholar] [CrossRef]
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion-a literature review. J. Spine Surg. 2020, 6, 752–761. [Google Scholar] [CrossRef]
- Cloward, R.B. The treatment of ruptured lumbar intervertebral discs: Criteria for spinal fusion. Am. J. Surg. 1953, 86, 145–151. [Google Scholar] [CrossRef]
- Tarpada, S.P.; Morris, M.T.; Burton, D.A. Spinal fusion surgery: A historical perspective. J. Orthop. 2017, 14, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Pelletier, M.H.; Mobbs, R.J.; Walsh, W.R. The design evolution of interbody cages in anterior cervical discectomy and fusion: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.W.; Robinson, R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J. Bone Jt. Surg. Am. 1958, 40, 607–624. [Google Scholar] [CrossRef]
- Robbins, S.; Lauryssen, C.; Songer, M.N. Use of Nanocrystalline Hydroxyapatite with Autologous BMA and Local Bone in the Lumbar Spine. A Retrospective CT Analysis of Posterolateral Fusion Results. Clin. Spine Surg. 2017, 30, E192–E197. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.G.; Cavanaugh, D.A.; Nunley, P.; Utter, P.A.; Kerr, E.; Wadhwa, R.; Stone, M. PEEK versus titanium cages in lateral lumbar interbody fusion: A comparative analysis of subsidence. Neurosurg. Focus 2020, 49, E10. [Google Scholar] [CrossRef]
- Rao, P.J.; Pelletier, M.H.; Walsh, W.R.; Mobbs, R.J. Spine interbody implants: Material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop. Surg. 2014, 6, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Massaad, E.; Fatima, N.; Kiapour, A.; Hadzipasic, M.; Shankar, G.M.; Shin, J.H. Polyetheretherketone Versus Titanium Cages for Posterior Lumbar Interbody Fusion: Meta-Analysis and Review of the Literature. Neurospine 2020, 17, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Iunes, E.A.; Barletta, E.A.; Barba Belsuzarri, T.A.; Onishi, F.J.; Cavalheiro, S.; Joaquim, A.F. Correlation Between Different Interbody Grafts and Pseudarthrosis After Anterior Cervical Discectomy and Fusion Compared with Control Group: Systematic Review. World Neurosurg. 2020, 134, 272–279. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Zhao, B.; Lu, X.D.; Zhao, Y.B.; Zhao, X.F.; Wang, X.N.; Zhou, R.T.; Qi, D.T.; Wang, W.X. Mid- and Long-Term Follow-Up Efficacy Analysis of 3D-Printed Interbody Fusion Cages for Anterior Cervical Discectomy and Fusion. Orthop. Surg. 2021, 13, 1969–1978. [Google Scholar] [CrossRef]
- Dietz, N.; Sharma, M.; Adams, S.; Alhourani, A.; Ugiliweneza, B.; Wang, D.; Nuño, M.; Drazin, D.; Boakye, M. Enhanced Recovery After Surgery (ERAS) for Spine Surgery: A Systematic Review. World Neurosurg. 2019, 130, 415–426. [Google Scholar] [CrossRef]
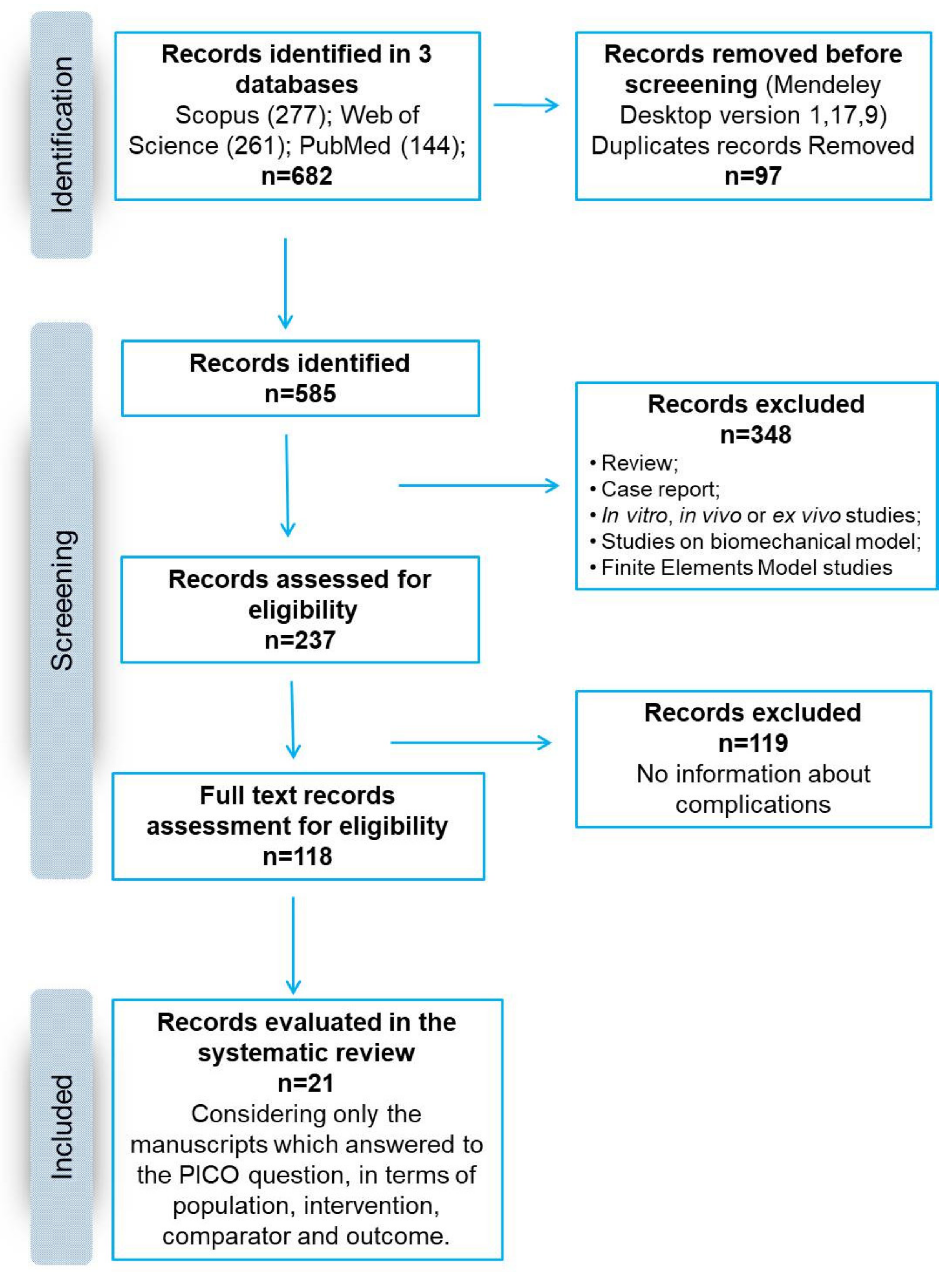


| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Study Type (f-up) | Cages (no. of pz) | Systemic and Local Complications | Fusion Results | Clinical Score Results | Ref. |
|---|---|---|---|---|---|
| Posterolateral lumbar interbody fusion (PLIF) 1-, 2- or 3-level PLIF | |||||
| Randomized study (24 mo) | PEEK cage (35 pz), ABG (34 pz) | PEEK cage and ABG: dural tears. PEEK cage: superficial wound infection, no cage loosening or breaking | PEEK cage and ABG: ↑ fusion rate, mean disc height | PEEK cage and ABG: ↓ pain, VAS score with good functional outcomes | [19] |
| Prospective, nonrandomized, controlled study (mean 32 mo) | PEEK cage + ABG (173 pz), Biocage (206 pz) | PEEK cage + ABG: similar operation time, blood loss, LOS, pseudoarthrosis, subsidence, delayed incision healing to Biocage | PEEK cage + ABG and Biocage: ↑ fusion rate. PEEK cage: ↓ mean height of intervertebral space recovery, height of intervertebral foramen recovery compared to Biocage | PEEK cage + ABG and Biocage: ↓ VAS, ODI | [20] |
| Prospective, uncontrolled study (12 mo) | PEEK + βTCP + BMA cage (34 pz) | Blood loss, transient paresis L5, dura leakage, migration of cage, seroma, inadequate fusion | ↑ fusion rate | ↓ ODI, VAS | [21] |
| Transforaminal lumbar interbody fusion (TLIF) 1-or multi-level TLIF | |||||
| Retrospective study (24 mo) | PEEK cage + ABG (40 pz), Ti cage + ABG (77 pz) | PEEK cage + ABG: pseudoarthrosis at 10 level. Ti cage + ABG: pseudoarthrosis at 16 level | PEEK cage + ABG: similar bone union rate to Ti cage + ABG | / | [22] |
| Observational, prospective, nonrandomized cohort study (24 mo) | PEEK + silicon cage added with Ti screw (22 pz) | LOS, pulmonary disease. Material failure in the dynamic portion, revision surgery, lumbar radiculopathy with no neurological deficit, misplaced pedicle screw and revision surgery, superficial wound infection, incidental durotomy | / | ↓ COMI, VAS scores | [23] |
| Randomised controlled clinical pilot trial (12 mo) | PEEK cage (20 pz), TiPEEK cage (20 pz) | PEEK cage: similar revision for pseudoarthrosis, loose pedicle screws, i.o. hematoma to TiPEEK cage. TiPEEK cage: persistent leg pain, p.o. wound infection | PEEK and TiPEEK cages: ↑ fusion rate, preservation of disc height in the fused or adjacent segments | PEEK and TiPEEK cages: ↓ ODI score, ↑ EQ-5D. TiPEEK cage: ↑ VAS leg pain compared to PEEK cage | [24] |
| Anterior lumbar interbody fusion (ALIF) 1-, 2-, 3-level ALIF | |||||
| Prospective, randomized, controlled clinical trial (12 mo) | PEEK + βTCP + BMA cage (50 pz) | Blood loss, paresis L5, hematoma, vessel lesions, migration of cage, pseudoarthrosis, inadequate fusion anteriorly | ↑ fusion | ↓ ODI, VAS | [25] |
| Prospective, uncontrolled study (mean 12 mo) | PEEK + rhBMP-2 cage (131 pz) | Minor complications. Major complications, prolonged pseudo-obstruction of the colon, DVT, bilateral pleural effusions, aspiration pneumonia, UTI. Pseudoarthrosis | ↑ interbody fusion | ↓ ODI, VAS, ↑ SF-36 PCS and SF-36 MCS | [26] |
| Extreme lateral interbody fusion (XLIF) | |||||
| Retrospective study (24 mo) | PEEK + βTCP + HA cage (25 pz), PEEK + rhBMP-2 cage (110 pz) | PEEK + rhBMP-2 cage: hematoma. PEEK + βTCP + HA cage: similar radiculopathy, subsidence, superficial wound infection to PEEK + rhBMP-2 cage | PEEK + rhBMP-2 cage: ↑ fusion rate compared to PEEK + βTCP + HA cage | PEEK + βTCP + HA and PEEK + rhBMP-2 cages: ↓ ODI, VAS, ↑ SF-36 PCS, SF-36 MCS | [27] |
| Anterior cervical discectomy and fusion (ACDF) 1- and 2-level ACDF | |||||
| Retrospective analytical observational cohort study | PEEK + ABG cage (30 pz), ABG (30 pz) | PEEK + ABG cage: ↓ operation time compared to ABG. ABG: donor site chronic pain, surgical wound infection, reoperation due to broken fixation system screw | PEEK + ABG cage and ABG: ↑ fusion rates, recovery of disc space height. PEEK + ABG cage: similar fusion rate as ABG | PEEK + ABG cage and ABG: ↑ clinical results | [28] |
| / (24 mo) | PEEK + ABG cage (29 pz), ABG (31 pz) | PEEK + ABG cage: ↓ operation time, blood loss, perioperative complications compared to ABG. PEEK + ABG cage: dysphagia. ABG: donor site pain, dysphagia, wound infections | PEEK + ABG cage and ABG: ↑ fusion rate. PEEK + ABG cage: similar fusion rate as ABG | PEEK + ABG cage and ABG: ↓ VAS, ↑ JOA score. PEEK + ABG cage: ↓ DSH than ABG | [29] |
| Prospective study (24 mo) | PEEK + ABG cage (28 pz) | 4-level ACDF: ↑ operation time, bleeding compared to 2- and 3-level ACDF. 2-, 3- and 4-level ACDF: Transient dysphagia, subsidence. 3- and 4-level ACDF: significant dysphagia, pseudoarthrosis rate, transient donor site pain | 2-, 3- and 4-level ACDF: ↑ solid fusion | 2-, 3- and 4-level ACDF: ↓ VAS, excellent and good results | [30] |
| Retrospective study (mean 29 mo) | Self-locking PEEK + bioceramic artificial bone cage with or without plate fixation (54 pz) | Mild dysphagia. Mild pseudoarthrosis | With and without plate fixation: ↑ fusion rate | With and without plate fixation: ↑ NDI, JOA | [31] |
| Prospective, single-blind randomized controlled study (24 mo) | PEEK + ABG cage (48 pz), Silicon nitride + blood cage (52 pz) | PEEK + ABG cage: similar operation time, blood loss, LOS, transient dysphagia, subsidence, incidental durotomy, recurrent symptomatic nerve root compression as silicon nitride + blood cage. PEEK + ABG cage: ↓ revision surgery at the adjacent level than silicon nitride + blood cage | PEEK + ABG and silicon nitride + blood cages: ↑ fusion rate | PEEK + ABG and silicon nitride + blood cages: ↑ NDI, SF36, patient perceived recovery, ↓ VAS | [32] |
| Retrospective study (mean 96.4 mo) | PEEK + ABG cage (47 pz), nHA/PA66 + ABG cage (51 pz) | PEEK + ABG cage: similar wound infection, subsidence to nHA/PA66 + ABG cage | PEEK + ABG and nHA/PA66 + ABG cages: ↑ fusion rate, segmental lordosis. PEEK + ABG cage: similar fusion rate as nHA/PA66 + ABG cage | PEEK + ABG and nHA/PA66 + ABG cages: ↑ JOA score, ↓ VAS score, good clinical outcome. | [33] |
| Prospective, randomized, controlled clinical study (24 mo) | PEEK + ABG cage (33 pz), PEEK + CS/DBM cage (35 pz) | PEEK + ABG cage: ↑ operation time, blood loos, total complication rate compared to PEEK + CS/DBM cage. PEEK + ABG cage: similar LOS, minor complications, hoarseness, superficial wound infection as PEEK + CS/DBM cage | PEEK + ABG and PEEK + CS/DBM cages: ↑ fusion rate. PEEK + ABG cage: similar fusion rate as PEEK + CS/DBM cage | PEEK + ABG and PEEK + CS/DBM cages: ↓ VAS, ↑ JOA score | [34] |
| Retrospective study (mean 30 mo) | PEEK + ABG cage (23 pz), PEEK + PolyBone cage (24 pz) | PEEK + ABG cage: similar operation time as PEEK + PolyBone cage | PEEK + ABG and PEEK + PolyBone cages: ↑ fusion rate. PEEK + PolyBone cage: ↓ disc height, ↑ time taken for fusion compared to PEEK + ABG cage | PEEK + ABG and PEEK + PolyBone cages: ↓ NDI, NRS score | [35] |
| Prospective, single-blind, randomized, controlled clinical study (12 mo) | PEEK + βTCP cage (32 pz), Acrylic cage (32 pz) | PEEK + βTCP cage: similar transient hoarseness, new degenerative changes at each level of the cervical spine, disk herniation at lower level compared to acrylic cage | PEEK + βTCP cage: ↓ fusion rate, disc space height compared to acrylic cage. PEEK + βTCP cage: similar subsidence as acrylic cage | PEEK + βTCP cage: ↓ clinical outcomes compared to acrylic cage | [36] |
| Retrospective chart review (median 12 mo) | PEEK + βTCP cage (107 pz), PEEK + rhBMP2 cage (84 pz) | PEEK + βTCP cage: ↓ 30-day readmission, oral steroids compared to PEEK + rhBMP2 cage. PEEK + βTCP cage: similar LOS, postoperative neurologic deficit, any dysphagia, ICU asPEEK + rhBMP2 cage. PEEK + βTCP cage: hardware failures. PEEK + βTCP cage: ↑ subsequent cervical spine surgery compared to PEEK + rhBMP2 cage | PEEK + βTCP cage: ↓ fusion rate compared to PEEK + rhBMP2 cage | / | [37] |
| Prospective single senior surgeon cohort study (mean 14.6 mo) | PEEK + Ti alloy + allograft cage (24 pz) | Without anterior plate fixation: pseudoarthrosis | With and without anterior plate fixation: ↑ fusion rate | With and without anterior plate fixation: ↑ MCS, ↓ VAS, good and excellent clinical outcomes | [38] |
| Retrospective cohort study (mean 24 mo) | Non-Plasmapore-coated Ti cage (42 pz), Ti cage coated with Plasmapore (30 pz) | None-Plasmapore-coated Ti cage: similar blood loss, operation time as Ti cage coated with Plasmapore | Non-Plasmapore-coated Ti cage and Ti cage coated with Plasmapore: ↑ solid fusion rate | / | [39] |
| Part A | Part B | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Size | Mean F-Up | Surgical Approach | Type of Study | Description of Diagnosis | Description of Surgical Technique | Description of p.o. Rehabilitation | Outcome Criteria | Procedure for Assessing Outcomes | Description of Subject Selection Process | ||
| [19] | 7 | 4 | 10 | 10 | 0 | 10 | 0 | 10 | 12 | 5 | 68 |
| [20] | 10 | 4 | 10 | 10 | 5 | 10 | 0 | 10 | 8 | 5 | 72 |
| [21] | 10 | 4 | 10 | 0 | 0 | 0 | 0 | 10 | 5 | 10 | 49 |
| [22] | 4 | 4 | 10 | 10 | 5 | 10 | 5 | 10 | 12 | 5 | 75 |
| [23] | 4 | 4 | 10 | 10 | 5 | 0 | 0 | 10 | 8 | 5 | 56 |
| [24] | 10 | 4 | 10 | 0 | 5 | 5 | 0 | 10 | 8 | 10 | 62 |
| [25] | 10 | 4 | 10 | 10 | 5 | 10 | 0 | 8 | 12 | 5 | 74 |
| [26] | 0 | 4 | 10 | 10 | 5 | 0 | 0 | 10 | 12 | 5 | 56 |
| [27] | 4 | 4 | 10 | 15 | 5 | 10 | 5 | 10 | 8 | 5 | 76 |
| [28] | 7 | 0 | 10 | 0 | 5 | 10 | 0 | 10 | 12 | 10 | 64 |
| [29] | 7 | 4 | 10 | 0 | 0 | 5 | 0 | 10 | 8 | 10 | 54 |
| [30] | 0 | 4 | 7 | 10 | 0 | 10 | 0 | 10 | 8 | 0 | 49 |
| [31] | 7 | 4 | 10 | 0 | 0 | 10 | 0 | 10 | 12 | 10 | 63 |
| [32] | 7 | 4 | 10 | 15 | 5 | 10 | 0 | 10 | 12 | 10 | 83 |
| [33] | 7 | 10 | 10 | 0 | 0 | 5 | 0 | 10 | 9 | 10 | 61 |
| [34] | 7 | 4 | 10 | 15 | 5 | 10 | 0 | 10 | 8 | 5 | 74 |
| [35] | 4 | 4 | 10 | 0 | 0 | 10 | 0 | 10 | 8 | 10 | 56 |
| [36] | 7 | 4 | 10 | 15 | 5 | 10 | 0 | 10 | 5 | 5 | 71 |
| [37] | 10 | 4 | 10 | 0 | 5 | 5 | 0 | 10 | 5 | 10 | 59 |
| [38] | 0 | 4 | 10 | 10 | 0 | 10 | 0 | 10 | 12 | 0 | 56 |
| [39] | 7 | 4 | 10 | 15 | 0 | 10 | 0 | 10 | 12 | 5 | 73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Sartori, M.; Griffoni, C.; Valacco, M.; Tedesco, G.; Davassi, P.F.; Gasbarrini, A.; Fini, M.; Barbanti Brodano, G. Complications in Spinal Fusion Surgery: A Systematic Review of Clinically Used Cages. J. Clin. Med. 2022, 11, 6279. https://doi.org/10.3390/jcm11216279
Veronesi F, Sartori M, Griffoni C, Valacco M, Tedesco G, Davassi PF, Gasbarrini A, Fini M, Barbanti Brodano G. Complications in Spinal Fusion Surgery: A Systematic Review of Clinically Used Cages. Journal of Clinical Medicine. 2022; 11(21):6279. https://doi.org/10.3390/jcm11216279
Chicago/Turabian StyleVeronesi, Francesca, Maria Sartori, Cristiana Griffoni, Marcelo Valacco, Giuseppe Tedesco, Paolo Francesco Davassi, Alessandro Gasbarrini, Milena Fini, and Giovanni Barbanti Brodano. 2022. "Complications in Spinal Fusion Surgery: A Systematic Review of Clinically Used Cages" Journal of Clinical Medicine 11, no. 21: 6279. https://doi.org/10.3390/jcm11216279
APA StyleVeronesi, F., Sartori, M., Griffoni, C., Valacco, M., Tedesco, G., Davassi, P. F., Gasbarrini, A., Fini, M., & Barbanti Brodano, G. (2022). Complications in Spinal Fusion Surgery: A Systematic Review of Clinically Used Cages. Journal of Clinical Medicine, 11(21), 6279. https://doi.org/10.3390/jcm11216279









