Pharmacological Interventions for Excessive Daytime Sleepiness in Adults with Narcolepsy: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Quality and Risk of Bias
2.4. Outcomes and Data Extraction
2.5. Statistical Analysis
3. Results
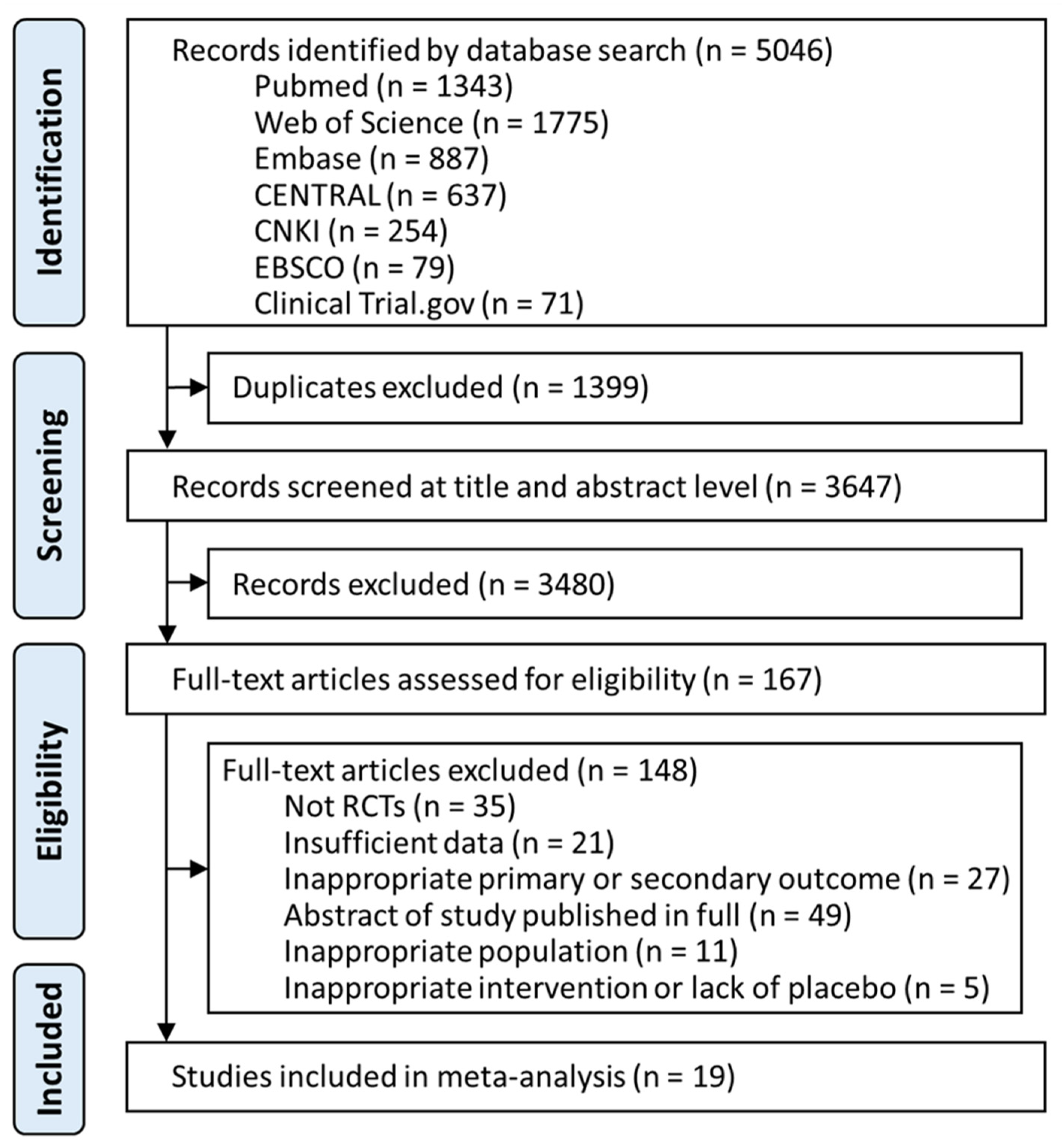
3.1. Study Selection and Baseline Characteristics
3.2. Primary Outcomes
3.2.1. Change in Epworth Sleepiness Scale
3.2.2. Change in Maintenance of Wakefulness Test
3.3. Secondary Outcomes
3.3.1. Change in Cataplexy Rate
3.3.2. Clinical Global Impression of Change
3.3.3. Adverse Events
3.4. Three-Dimensional Clustered Ranking Plot
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scammell, T.E. Narcolepsy. N. Engl. J. Med. 2015, 373, 2654–2662. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, W.; Koepsell, T.D.; Ton, T.G.; Hendrickson, A.F.; Van Belle, G. The epidemiology of narcolepsy. Sleep 2007, 30, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Kallweit, U.; Vignatelli, L.; Plazzi, G.; Lecendreux, M.; Baldin, E.; Dolenc-Groselj, L.; Jennum, P.; Khatami, R.; Manconi, M.; et al. European guideline and expert statements on the management of narcolepsy in adults and children. Eur. J. Neurol. 2021, 28, 2815–2830. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.; Broughton, R. Scheduled naps in the management of daytime sleepiness in narcolepsy-cataplexy. Sleep 1993, 16, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Fowler, J.S.; Logan, J.; Alexoff, D.; Zhu, W.; Telang, F.; Wang, G.J.; Jayne, M.; Hooker, J.M.; Wong, C.; et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: Clinical implications. Jama 2009, 301, 1148–1154. [Google Scholar] [CrossRef] [Green Version]
- Wisor, J.P.; Nishino, S.; Sora, I.; Uhl, G.H.; Mignot, E.; Edgar, D.M. Dopaminergic role in stimulant-induced wakefulness. J. Neurosci. 2001, 21, 1787–1794. [Google Scholar] [CrossRef]
- Malhotra, A.; Shapiro, C.; Pepin, J.-L.; Hedner, J.; Ahmed, M.; Foldvary-Schaefer, N.; Strollo, P.J.; Mayer, G.; Sarmiento, K.; Baladi, M.; et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep 2020, 43, zsz220. [Google Scholar] [CrossRef]
- Drakatos, P.; Lykouras, D.; D’Ancona, G.; Higgins, S.; Gildeh, N.; Macavei, R.; Rosenzweig, I.; Steier, J.; Williams, A.J.; Muza, R.; et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med. 2017, 35, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, X.; Yang, S.; Wang, T.; Xu, Z.; Xu, J.; Gao, H.; Chen, G. Pitolisant versus placebo for excessive daytime sleepiness in narcolepsy and obstructive sleep apnea: A meta-analysis from randomized controlled trials. Pharmacol. Res. 2021, 167, 105522. [Google Scholar] [CrossRef]
- Junnarkar, G.; Allphin, C.; Profant, J.; Steininger, T.L.; Chen, C.; Zomorodi, K.; Skowronski, R.; Black, J. Development of a lower-sodium oxybate formulation for the treatment of patients with narcolepsy and idiopathic hypersomnia. Expert Opin. Drug Discov. 2022, 17, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Lehert, P.; Szoeke, C. Comparison of modafinil and pitolisant in narcolepsy: A non-inferiority meta-analytical approach. Drugs Context 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Subedi, R.; Singh, R.; Thakur, R.K.; Bibek, K.C.; Jha, D.; Ray, B.K. Efficacy and safety of solriamfetol for excessive daytime sleepiness in narcolepsy and obstructive sleep apnea: A systematic review and meta-analysis of clinical trials. Sleep Med. 2020, 75, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Lehert, P.; Falissard, B. Multiple treatment comparison in narcolepsy: A network meta-analysis. Sleep 2018, 41, zsy185. [Google Scholar] [CrossRef] [Green Version]
- Watt, J.; Del Giovane, C. Network Meta-Analysis. Methods Mol. Biol. 2022, 2345, 187–201. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Elbourne, D.R.; Altman, D.G.; Higgins, J.; Curtin, F.; Worthington, H.; Vail, A. Meta-analyses involving cross-over trials: Methodological issues. Int. J. Epidemiol. 2002, 31, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Arand, D.; Bonnet, M.; Hurwitz, T.; Mitler, M.; Rosa, R.; Sangal, R. The clinical use of the MSLT and MWT. Sleep 2005, 28, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Arzi, L.; Shreter, R.; El-Ad, B.; Peled, R.; Pillar, G. Forty- versus 20-minute trials of the maintenance of wakefulness test regimen for licensing of drivers. J. Clin. Sleep Med. 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef]
- Krahn, U.; Binder, H.; König, J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med. Res. Methodol. 2013, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Rücker, G.; Krahn, U.; König, J.; Efthimiou, O.; Davies, A.; Papakonstantinou, T.; Schwarzer, G. Netmeta: Network Meta-Analysis Using Frequentist Methods. 2019. Available online: https://github.com/guido-s/netmeta (accessed on 1 October 2020).
- Bogan, K.R.; Thorpy, M.J.; Dauvilliers, Y.; Partinen, M.; Villegas, R.d.; Foldvary-Schaefer, N.; Skowronski, R.; Tang, L.; Skobieranda, F.; Šonka, K. Efficacy and safety of calcium, magnesium, potassium, and sodium oxybates (lower-sodium oxybate [LXB]; JZP-258) in a placebo-controlled, double-blind, randomized withdrawal study in adults with narcolepsy with cataplexy. Sleep 2021, 44, zsaa206. [Google Scholar] [CrossRef]
- Bogan, R.K.; Feldman, N.; Emsellem, H.A.; Rosenberg, R.; Lu, Y.; Bream, G.; Khayrallah, M.; Lankford, D.A. Effect of oral JZP-110 (ADX-N05) treatment on wakefulness and sleepiness in adults with narcolepsy. Sleep Med. 2015, 16, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Ruoff, C.; Swick, T.J.; Doekel, R.; Emsellem, H.A.; Feldman, N.T.; Rosenberg, R.; Bream, G.; Khayrallah, M.A.; Lu, Y.; Black, J. Effect of Oral JZP-110 (ADX-N05) on Wakefulness and Sleepiness in Adults with Narcolepsy: A Phase 2b Study. Sleep 2016, 39, 1379–1387. [Google Scholar] [CrossRef]
- Thorpy, M.J.; Shapiro, C.; Mayer, G.; Corser, B.C.; Emsellem, H.; Plazzi, G.; Chen, D.; Carter, L.P.; Wang, H.; Lu, Y.; et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann. Neurol. 2019, 85, 359–370. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Bassetti, C.; Lammers, G.J.; Arnulf, I.; Mayer, G.; Rodenbeck, A.; Lehert, P.; Ding, C.-L.; Lecomte, J.-M.; Schwartz, J.-C. Pitolisant versus placebo or modafinil in patients with narcolepsy: A double-blind, randomised trial. Lancet Neurol. 2013, 12, 1068–1075. [Google Scholar] [CrossRef]
- Kollb-Sielecka, M.; Demolis, P.; Emmerich, J.; Markey, G.; Salmonson, T.; Haas, M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: Summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017, 33, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, Z.; Dauvilliers, Y.; Mikhaylov, V.; Poverennova, I.; Krylov, S.; Jankovic, S.; Sonka, K.; Lehert, P.; Lecomte, I.; Lecomte, J.-M.; et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017, 16, 200–207. [Google Scholar] [CrossRef]
- Fry, J.M.; US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. Ann. Neurol. 1998, 43, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.T.; US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy: US Modafinil in Narcolepsy Multicenter Study Group. Neurology 2000, 54, 1166–1175. [Google Scholar]
- Moldofsky, H.; Broughton, R.J.; Hill, J.D. A randomized trial of the long-term, continued efficacy and safety of modafinil in narcolepsy. Sleep Med. 2000, 1, 109–116. [Google Scholar] [CrossRef]
- Saletu, M.; Anderer, P.; Saletu-Zyhlarz, G.M.; Mandl, M.; Saletu, B.; Zeitlhofer, J. Modafinil improves information processing speed and increases energetic resources for orientation of attention in narcoleptics: Double-blind, placebo-controlled ERP studies with low-resolution brain electromagnetic tomography (LORETA). Sleep Med. 2009, 10, 850–858. [Google Scholar] [CrossRef]
- Xyrem®; International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J. Clin. Sleep Med. 2005, 1, 391–397. [Google Scholar] [CrossRef]
- US Xyrem® Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 2002, 25, 42–49. [Google Scholar]
- Black, J.; Houghton, W.C. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep 2006, 29, 939–946. [Google Scholar] [CrossRef]
- Harsh, J.R.; Hayduk, R.; Rosenberg, R.; Wesnes, K.A.; Walsh, J.K.; Arora, S.; Niebler, G.E.; Roth, T. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr. Med. Res. Opin. 2006, 22, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Xyrem® International Study Group. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: A double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005, 6, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lammers, G.J.; Arends, J.; Declerck, A.C.; Ferrari, M.D.; Schouwink, G.; Troost, J. Gammahydroxybutyrate and narcolepsy: A double-blind placebo-controlled study. Sleep 1993, 16, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Scrima, L.; Hartman, P.G.; Johnson, F.H., Jr.; Hiller, F.C. Efficacy of gamma-hydroxybutyrate versus placebo in treating narcolepsy-cataplexy: Double-blind subjective measures. Biol. Psychiatry 1989, 26, 331–343. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Shapiro, C.; Mayer, G.; Lammers, G.J.; Emsellem, H.; Plazzi, G.; Chen, D.; Carter, L.P.; Lee, L.; Black, J.; et al. Solriamfetol for the Treatment of Excessive Daytime Sleepiness in Participants with Narcolepsy with and without Cataplexy: Subgroup Analysis of Efficacy and Safety Data by Cataplexy Status in a Randomized Controlled Trial. CNS Drugs 2020, 34, 773–784. [Google Scholar] [CrossRef]
- Maski, M.K.; Trotti, L.M.; Kotagal, S.; Auger, R.R.; Swick, T.J.; Rowley, J.A.; Hashmi, M.S.D.; Watson, N.F. Treatment of Central Disorders of Hypersomnolence: An American Academy of Sleep Medicine Systematic Review, Meta-analysis, and GRADE Assessment. J. Clin. Sleep Med. 2021, 17, 1895–1945. [Google Scholar] [CrossRef]
- Xu, X.-M.; Wei, Y.-D.; Liu, Y.; Li, Z.-X. Gamma-hydroxybutyrate (GHB) for narcolepsy in adults: An updated systematic review and meta-analysis. Sleep Med. 2019, 64, 62–70. [Google Scholar] [CrossRef]
- Emsellem, H.A.; Thorpy, M.J.; Lammers, G.J.; Shapiro, C.M.; Mayer, G.; Plazzi, G.; Chen, D.; Carter, L.P.; Villa, K.F.; Lee, L.; et al. Measures of functional outcomes, work productivity, and quality of life from a randomized, phase 3 study of solriamfetol in participants with narcolepsy. Sleep Med. 2020, 67, 128–136. [Google Scholar] [CrossRef]
- Tadrous, R.; O’Rourke, D.; Mockler, D.; Broderick, J. Health-related quality of life in narcolepsy: A systematic review and meta-analysis. J. Sleep Res. 2021, 30, e13383. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products; Guidance for Industry; US Food and Drug Administration: Silver Spring, MD, USA, 2019.



| Change in ESS | ||||||
|---|---|---|---|---|---|---|
| Solriamfetol | ||||||
| −1.39 (−3.10–0.31) | Modafinil | |||||
| −1.76 (−4.27–0.74) | −0.37 (−2.73–1.99) | Lower-sodium oxybate | ||||
| −2.56 (−4.62–−0.51) | −1.17 (−3.04–0.70) | −0.80 (−3.42–1.82) | Sodium oxybate | |||
| −2.88 (−4.89–−0.88) | −1.49 (−3.07–0.09) | −1.12 (−3.70–1.46) | −0.32 (−2.46–1.82) | Pitolisant | ||
| −4.76 (−6.11–−3.42) | −3.37 (−4.42–−2.33) | −3.00 (−5.11–−0.89) | −2.20 (−3.75–−0.65) | −1.88 (−3.36–−0.40) | Placebo | |
| Change in MWT | ||||||
| Solriamfetol | ||||||
| 0.39 (−0.17–0.94) | Armodafinil | |||||
| 0.42 (0.05–0.79) | 0.03 (−0.48–0.54) | Modafinil | ||||
| 0.42 (−0.02–0.87) | 0.03 (−0.53–0.59) | 0.00 (−0.35–0.35) | Sodium oxybate | |||
| 0.45 (0.02–0.88) | 0.06 (−0.49–0.62) | 0.03 (−0.32–0.38) | 0.03 (−0.41–0.47) | Pitolisant | ||
| 0.97 (0.67–1.28) | 0.58 (0.12–1.05) | 0.55 (0.34–0.77) | 0.55 (0.23–0.87) | 0.52 (0.22–0.83) | Placebo | |
| Change in cataplexy rate | ||||||
| Pitolisant | ||||||
| −0.09 (−0.84–0.67) | Lower-sodium oxybate | |||||
| −0.32 (−0.86–0.21) | −0.24 (−0.97–0.49) | Sodium oxybate | ||||
| −0.53 (−1.19–0.13) | −0.44 (−1.35–0.48) | −0.20 (−0.95–0.55) | Modafinil | |||
| −0.72 (−1.46–0.01) | −0.64 (−1.52–0.25) | −0.40 (−1.11–0.31) | −0.20 (−1.10–0.71) | Armodafinil | ||
| −0.72 (−1.13–−0.32) | −0.64 (−1.27–0.00) | −0.40 (−0.76–−0.04) | −0.20 (−0.86–0.46) | −0.00 (−0.62–0.62) | Placebo | |
| CGI-C | ||||||
| Lower-sodium oxybate | ||||||
| 1.76 (0.80–3.85) | Armodafinil | |||||
| 1.83 (0.94–3.55) | 1.04 (0.57–1.90) | Sodium oxybate | ||||
| 1.90 (0.98–3.66) | 1.08 (0.59–1.96) | 1.04 (0.68–1.59) | Solriamfetol | |||
| 2.27 (1.14–4.52) | 1.29 (0.68–2.42) | 1.24 (0.78–1.98) | 1.19 (0.75–1.91) | Pitolisant | ||
| 2.42 (1.28–4.57) | 1.37 (0.78–2.43) | 1.32 (0.91–1.91) | 1.27 (0.87–1.87) | 1.07 (0.74–1.54) | Modafinil | |
| 3.82 (2.12–6.88) | 2.17 (1.30–3.64) | 2.09 (1.53–2.84) | 2.02 (1.50–2.71) | 1.69 (1.17–2.43) | 1.58 (1.24–2.02) | Placebo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, P.-Y.; Kuo, C.-Y.; Lin, M.-H.; Chang, Y.-J.; Hung, C.-C. Pharmacological Interventions for Excessive Daytime Sleepiness in Adults with Narcolepsy: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2022, 11, 6302. https://doi.org/10.3390/jcm11216302
Chien P-Y, Kuo C-Y, Lin M-H, Chang Y-J, Hung C-C. Pharmacological Interventions for Excessive Daytime Sleepiness in Adults with Narcolepsy: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2022; 11(21):6302. https://doi.org/10.3390/jcm11216302
Chicago/Turabian StyleChien, Po-Yu, Chan-Yen Kuo, Meng-Hsuan Lin, Yao-Jen Chang, and Chin-Chuan Hung. 2022. "Pharmacological Interventions for Excessive Daytime Sleepiness in Adults with Narcolepsy: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 11, no. 21: 6302. https://doi.org/10.3390/jcm11216302








