Analysis of the Lack of Follow-Up of Bariatric Surgery Patients: Experience of a Reference Center
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.2.1. Preoperative Data
Clinical Data
Surgery Procedure
Deprivation Index
Geographical Health Accessibility Index
2.2.2. Postoperative Data
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Results for COX Modeling of Follow Up Interruption
3.1.1. Population Description
3.1.2. Follow Up Variables
3.1.3. Univariates Analysis
3.1.4. Multivariate Analysis
3.2. Results for Multilevel Modeling Irregular Follow-Up
3.2.1. Population Description
3.2.2. Univariates Analysis
3.2.3. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| IF− a (n c = 433), % | IF+ b (n = 1116), % | ||||
|---|---|---|---|---|---|
| Variables | p d | ||||
| Age | |||||
| Continuous years means ± SD e | 433 | 43.55 ± 12.12 | 1116 | 43.03 ± 11.58 | 0.5363 |
| Gender | |||||
| Female | 362 | 83.6 | 859 | 76.97 | 0.0041 |
| Male | 71 | 16.4 | 257 | 23.03 | |
| EDI f | |||||
| Continuous, means ± SD | 423 | −1.10± 4.93 | 1095 | 0.82 ± 4.54 | 0.486 |
| Missing data (%) | 10 | 2.31 | 21 | 1.88 | 0.5896 |
| SCALE g | |||||
| Continuous, means ± SD | 413 | 4.16 ± 4.86 | 1079 | −3.69 ± 4.92 | 0.0783 |
| Missing data (%) | 20 | 4.62 | 37 | 3.32 | 0.2214 |
| Years of intervention | |||||
| 2012 | 20 | 4.62 | 138 | 12.37 | <0.0001 |
| 2013 | 21 | 4.85 | 141 | 12.63 | |
| 2014 | 16 | 3.7 | 163 | 14.61 | |
| 2015 | 43 | 9.93 | 175 | 15.68 | |
| 2016 | 78 | 18.01 | 138 | 12.37 | |
| 2017 | 71 | 16.4 | 144 | 12.9 | |
| 2018 | 83 | 19.17 | 141 | 12.63 | |
| 2019 | 101 | 23.33 | 76 | 6.81 | |
| Surgical Procedure | |||||
| Gastric Bypass | 273 | 63.05 | 752 | 67.38 | 0.1056 |
| Sleeve Gastrectomy | 160 | 36.95 | 364 | 32.62 | |
| Revisional Surgery | |||||
| No | 381 | 87.99 | 1000 | 89.61 | 0.359 |
| Yes | 52 | 12.01 | 116 | 10.39 | |
| Preoperative BMI h | |||||
| Continuous Kg/m² means ± SD | 433 | 42.21 ± 6.91 | 1116 | 42.17 ± 6.24 | 0.4701 |
| Preoperative weight loss | |||||
| Continuous Kg means ± SD | 433 | 9.45 ± 9.00 | 1116 | 10.02 ± 9.01 | 0.1722 |
| ASA I | |||||
| 2 | 304 | 70.21 | 859 | 76.97 | 0.0057 |
| 3 | 129 | 29.79 | 257 | 23.03 | |
| Sleep apnea syndrome | |||||
| No | 216 | 49.88 | 643 | 57.62 | 0.006 |
| Yes | 217 | 50.12 | 473 | 42.38 | |
| Arterial hypertension | |||||
| No | 281 | 64.9 | 740 | 66.31 | 0.5987 |
| Yes | 152 | 35.1 | 376 | 33.69 | |
| Dyslipidaemia | |||||
| No | 299 | 69.05 | 824 | 73.84 | 0.0586 |
| Yes | 134 | 30.95 | 292 | 26.16 | |
| Diabetes | |||||
| No | 312 | 72.06 | 856 | 76.7 | 0.0655 |
| Yes | 121 | 27.94 | 260 | 23.6 | |
Appendix B
| Times Follow Up (Months) | 1 | 3 | 6 | 12 | 18 | 24 | 36 | 48 | 60 |
|---|---|---|---|---|---|---|---|---|---|
| Cumulative rate (%) | 0.001 | 0.03 | 0.05 | 0.14 | 0.24 | 0.32 | 0.51 | 0.58 | 0.67 |
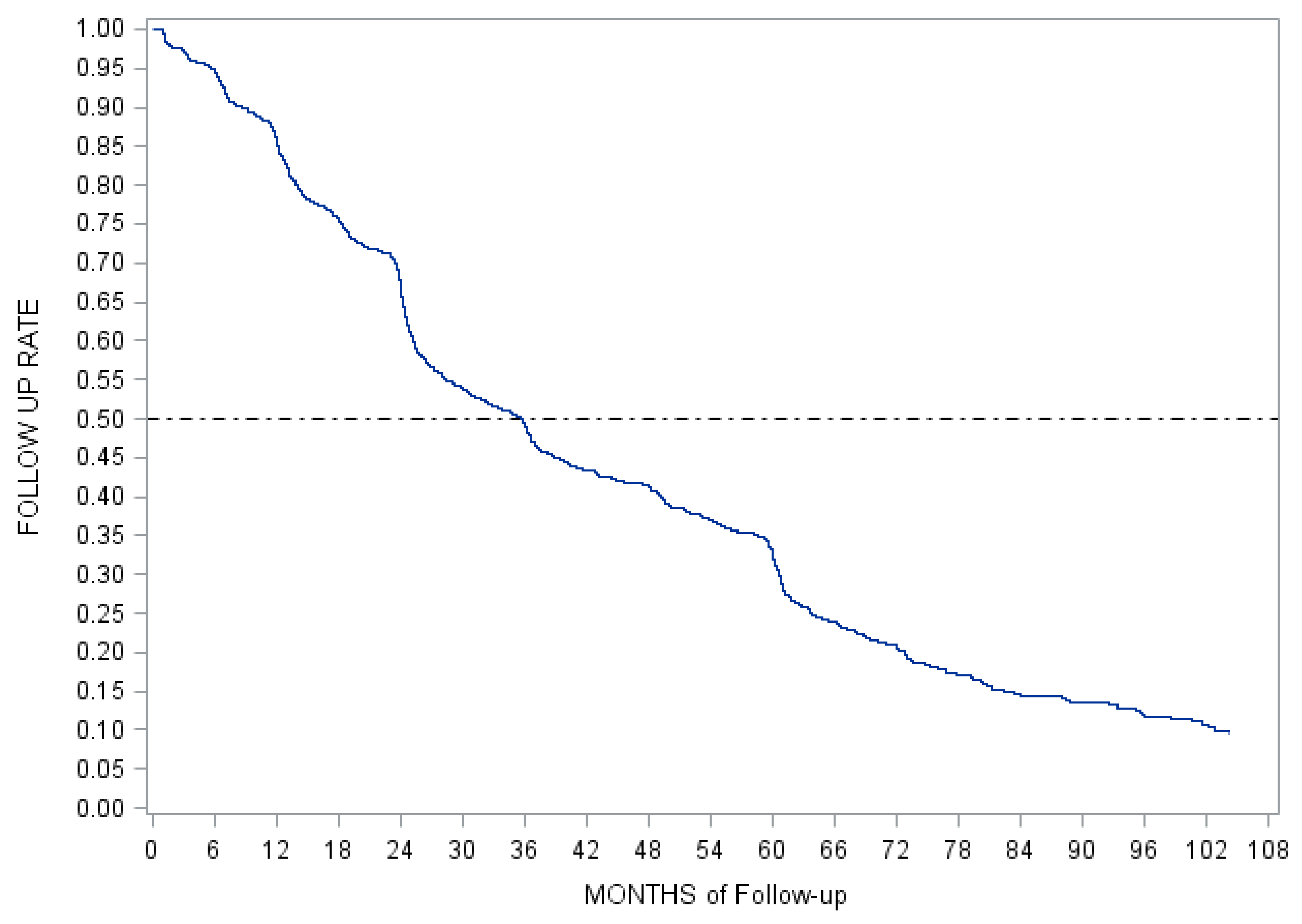
Appendix C
| n a | % | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR b | CI c | p d | HR b | CI c | p d | ||
| Age | ||||||||
| Continuous, years | 1549 | 100 | 0.997 | [0.992–1.002] | 0.3142 | 0.999 | [0.994–1.005] | 0.8603 |
| Gender | ||||||||
| Female | 1221 | 78.83 | Ref. | [1.092–1.443] | 0.0014 | Ref. | [1.146–1.527] | 0.0001 |
| Male | 328 | 21.17 | 1.255 | 1.323 | ||||
| EDI e | ||||||||
| Continuous, means ± SD | 1518 | 98 | 0.993 | [0.980–1.006] | 0.3115 | |||
| Missing data (%) | 31 | 2 | ||||||
| Scale f | ||||||||
| Continuous, means ± SD | 1492 | 96.32 | 1.008 | [0.995–1.020] | 0.2248 | |||
| Missing data (%) | 57 | 3.68 | ||||||
| Years of intervention | ||||||||
| Continuous | 1549 | 100 | 1.044 | [1.015–1.077] | 0.0035 | 1.043 | [1.012–1.076] | 0.0068 |
| Surgical Procedure | ||||||||
| Gastric Bypass | 1025 | 66.17 | Ref. | [0.900–1.157] | 0.7496 | |||
| Sleeve Gastrectomy | 524 | 33.83 | 1.021 | |||||
| Revisional Surgery | ||||||||
| No | 1381 | 89.15 | Ref. | [0.600–0.882] | 0.0012 | Ref. | [0.619–0.916] | 0.0045 |
| Yes | 168 | 10.85 | 0.727 | 0.753 | ||||
| Pre-Operative BMI | ||||||||
| Continuous, Kg/m² | 1549 | 100 | 0.99 | [0.981–0.999] | 0.0359 | 0.995 | [0.985–1.006] | 0.3723 |
| Preoperative weight loss | ||||||||
| Continuous Kg | 1549 | 100 | 1 | [0.994–1.007] | 0.909 | |||
| ASA | ||||||||
| 2 | 1163 | 75.08 | Ref. | [0.770–1.018] | 0.0875 | Ref. | [0.749–1.037] | 0.1282 |
| 3 | 386 | 24.92 | 0.886 | 0.881 | ||||
| Sleep apnea syndrome | ||||||||
| No | 859 | 55.46 | Ref. | [0.880–1.116] | 0.8833 | |||
| Yes | 690 | 44.54 | 0.991 | |||||
| Arterial hypertension | ||||||||
| No | 1021 | 65.91 | Ref. | [0.843–1.081] | 0.4664 | |||
| Yes | 528 | 34.09 | 0.955 | |||||
| Dyslipidaemia | ||||||||
| No | 1123 | 72.5 | Ref. | [0.847–1.106] | 0.628 | |||
| Yes | 426 | 27.5 | 0.968 | |||||
| Diabetes | ||||||||
| No | 1168 | 75.4 | Ref. | [0.784–1.035] | 0.1405 | Ref. | [0.770–1.043] | 0.1584 |
| Yes | 381 | 24.6 | 0.901 | 0.897 | ||||
Appendix D
| RF+ a (n c = 8951, 92.13) | RF− b (n = 756, 7.87) | ||||
|---|---|---|---|---|---|
| Variables | p d | ||||
| Age | |||||
| Continuous years, means ± SD | 8951 | 43.39 ± 11.41 | 756 | 43.80 ± 11.65 | 0.417 |
| Gender | |||||
| Female | 7118 | 80.42 | 594 | 78.57 | 0.2201 |
| Male | 1733 | 19.58 | 162 | 21.43 | |
| EDI e | |||||
| Continuous, means ± SD | 8690 | 1.19 ± 4.96 | 740 | 1.05 ± 4.55 | 0.9209 |
| Missing data (%) | 161 | 1.82 | 16 | 2.12 | 0.5595 |
| SCALE f | |||||
| Continuous, means ± SD | 8534 | −4.15 ± 4.88 | 733 | −3.81 ± 4.81 | 0.0557 |
| Missing data (%) | 317 | 3.58 | 23 | 3.04 | 0.4412 |
| Years of intervention | |||||
| 2012 | 962 | 10.87 | 83 | 10.98 | 0.008 |
| 2013 | 1017 | 11.49 | 69 | 9.13 | |
| 2014 | 972 | 10.98 | 94 | 12.43 | |
| 2015 | 1146 | 12.95 | 126 | 16.67 | |
| 2016 | 1396 | 15.77 | 120 | 15.87 | |
| 2017 | 1235 | 13.95 | 110 | 14.55 | |
| 2018 | 1216 | 13.74 | 100 | 13.23 | |
| 2019 | 907 | 10.25 | 54 | 7.14 | |
| Surgery Procedure | |||||
| Gastric By-pass | 5666 | 64.02 | 519 | 67.2 | 0.0799 |
| Sleeve Gastrectomy | 3185 | 35.98 | 252 | 32.8 | |
| Revisional Surgery | |||||
| No | 7474 | 84.44 | 672 | 88.89 | 0.0011 |
| Yes | 1377 | 15.56 | 84 | 11.11 | |
| Preoperative BMI | |||||
| Continuous Kg/m², means ± SD | 8 951 | 42.85 ± 7.16 | 756 | 41.93 ± 6.12 | 0.0072 |
| Preoperative weight loss | |||||
| Continuous Kg, means ± SD | 8951 | 10.06 ± 9.64 | 756 | 10.07 ± 9.27 | 0.8696 |
| ASA | |||||
| 2 | 6389 | 72.18 | 564 | 74.6 | 0.1533 |
| 3 | 2462 | 27.82 | 193 | 25.4 | |
| Sleep apnea syndrome | |||||
| No | 4858 | 54.89 | 414 | 54.76 | 0.9473 |
| Yes | 3993 | 45.11 | 342 | 45.24 | |
| Arterial hypertension | |||||
| No | 5685 | 64.23 | 480 | 63.49 | 0.6846 |
| Yes | 3166 | 35.77 | 276 | 36.51 | |
| Dyslipidaemia | |||||
| No | 6329 | 71.51 | 546 | 72.22 | 0.6752 |
| Yes | 2522 | 28.49 | 210 | 27.78 | |
| Diabetes | |||||
| No | 6575 | 74.29 | 562 | 74.34 | 0.9744 |
| Yes | 2276 | 25.71 | 194 | 25.66 | |
| TWL g | |||||
| Continuous, means ± SD | 8658 | 23.42 ± 10.75 | 737 | 27.87 ± 10.55 | <0.0001 |
| Missing data (%) | 193 | 2.18 | 19 | 2.51 | 0.5501 |
Appendix E
| n a | % | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | OR b | CI c | p d | OR b | CI c | p d | ||
| Age | ||||||||
| Continuous, years | 9607 | 100 | 1.003 | [0.997–1.010] | 0.3467 | 1.01 | [1.002–1.017] | 0.0086 |
| Gender | ||||||||
| Female | 7712 | 80.27 | Ref. | [0.934–1.343] | 0.2206 | Ref. | [1.047–1.545] | 0.0153 |
| Male | 1895 | 19.73 | 1.120 | 1.272 | ||||
| EDI e | ||||||||
| Continuous | 9430 | 98.16 | 0.994 | [0.979–1.010] | 0.4608 | |||
| Missing data (%) | 177 | 1.84 | ||||||
| SCALE f | ||||||||
| Continuous | 9267 | 96.46 | 1.014 | [0.998–1.030] | 0.0779 | 1.011 | [0.995–1.027] | 0.197 |
| Missing data (%) | 340 | 3.54 | ||||||
| Years of intervention | ||||||||
| Continuous | 9607 | 100 | 0.98 | [0.947–1.014] | 0.2399 | 0.986 | [0.950–1.022] | 0.4372 |
| Surgery Procedure | ||||||||
| Gastric By-pass | 6174 | 64.27 | Ref. | [0.742–1.017] | 0.0803 | Ref. | [0.943–1.349] | 0.1878 |
| Sleeve Gastrectomy | 3433 | 35.73 | 0.868 | 1.128 | ||||
| Revisional Surgery | ||||||||
| No | 8146 | 84.79 | Ref. | [0.537–0.858] | 0.0012 | Ref. | [0.543–0.922] | 0.0106 |
| Yes | 1461 | 15.21 | 0.678 | 0.707 | ||||
| Preoperative BMI | ||||||||
| Continuous, Kg/m² | 9607 | 100 | 0.98 | [0.969–0.991] | 0.0006 | 0.985 | [0.972–0.998] | 0.0263 |
| Preoperative weight loss | ||||||||
| Continuous Kg | 9607 | 100 | 1 | [0.993–1.008] | 0.9653 | |||
| ASA | ||||||||
| 2 | 6953 | 72.37 | Ref. | [0.745–1.048] | 0.1538 | Ref. | [0.774–1.159] | 0.5993 |
| 3 | 2654 | 27.63 | 0.883 | 0.947 | ||||
| Sleep apnea syndrome | ||||||||
| No | 5272 | 54.88 | Ref. | [0.866–1.167] | 0.9473 | |||
| Yes | 4335 | 45.12 | 1.005 | |||||
| Arterial hypertension | ||||||||
| No | 6155 | 64.17 | Ref. | [0.885–1.1205] | 0.6847 | |||
| Yes | 3442 | 35.83 | 1.032 | |||||
| Dyslipidaemia | ||||||||
| No | 6875 | 71.56 | Ref. | [0.818–1.139] | 0.6753 | |||
| Yes | 2732 | 28.44 | 0.965 | |||||
| Diabetes | ||||||||
| No | 7137 | 74.29 | Ref. | [0.841–1.182] | 0.9744 | |||
| Yes | 2470 | 25.71 | 0.997 | |||||
| TWL g | ||||||||
| Continuous | 9395 | 97.8 | 1.038 | [1.030–1.045] | <0.0001 | 1.04 | [1.033–1.048] | <0.0001 |
| Missing data | 212 | 2.21 | ||||||
References
- Kluge, H.H.P. WHO European Regional Obesity Report. 2022. Available online: https://www.euro.who.int/en/publications/abstracts/who-european-regional-obesity-report-2022 (accessed on 19 May 2022).
- Forte Progression de l’Obésité en France en 2020. Available online: https://liguecontrelobesite.org/actualite/forte-progression-de-lobesite-en-france-en-2020/ (accessed on 20 May 2022).
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.P.; Feigelson, H.S.; Koebnick, C.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arteburn, D.E. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann. Surg. 2019, 269, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bailly, L.; Fabre, R.; Pradier, C.; Iannelli, A. Colorectal Cancer Risk Following Bariatric Surgery in a Nationwide Study of French Individuals with Obesity. JAMA Surg. 2020, 155, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Direction de la Recherche, des Études, de l’Évaluation et des Statistiques. Chirurgie de l’Obésité: 20 Fois Plus d’Interventions Depuis 1997. Available online: https://drees.solidarites-sante.gouv.fr/publications/etudes-et-resultats/chirurgie-de-lobesite-20-fois-plus-dinterventions-depuis-1997 (accessed on 20 May 2022).
- HAS. Obésité: Prise en Charge Chirurgicale chez l’Adulte. Haute Autorité de Santé. Available online: https://www.has-sante.fr/jcms/c_765529/fr/obesite-prise-en-charge-chirurgicale-chez-l-adulte (accessed on 20 May 2022).
- Thereaux, J.; Lesuffleur, T.; Czernichow, S.; Basdevant, A.; Msika, S.; Nocca, D.; Millat, B.; Fagot-Campagna, A. Long-term adverse events after sleeve gastrectomy or gastric bypass: A 7-year nationwide, observational, population-based, cohort study. Lancet Diabetes Endocrinol. 2019, 7, 786–795. [Google Scholar] [CrossRef]
- Bjørklund, G.; Semenova, Y.; Pivina, L.; Costea, D.O. Follow-up after bariatric surgery: A review. Nutrition 2020, 78, 110831. [Google Scholar] [CrossRef]
- Castaneda, D.; Popov, V.B.; Wander, P.; Thompson, C.C. Risk of Suicide and Self-harm Is Increased After Bariatric Surgery—A Systematic Review and Meta-analysis. Obes. Surg. 2019, 29, 322–333. [Google Scholar] [CrossRef]
- King, W.C.; Chen, J.Y.; Courcoulas, A.P.; Dakin, G.F.; Engel, S.G.; Flum, D.R.; Hinojosa, M.W.; Kalarchian, M.A.; Mattar, S.G.; Mitchell, J.E.; et al. Alcohol and other substance use after bariatric surgery: Prospective evidence from a U.S. multicenter cohort study. Surg. Obes. Relat. Dis. 2017, 13, 1392–1402. [Google Scholar] [CrossRef]
- Higa, K.; Ho, T.; Tercero, F.; Yunus, T.; Boone, K.B. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg. Obes. Relat. Dis. 2011, 7, 516–525. [Google Scholar] [CrossRef]
- Higa, K.D.; Himpens, J. The reality of long-term follow-up of bariatric/metabolic surgery patients—A conundrum. JAMA Surg. 2018, 153, 435. [Google Scholar] [CrossRef]
- Clapp, B.; Wynn, M.; Martyn, C.; Foster, C.; O’Dell, M.; Tyroch, A. Long term (7 or more years) outcomes of the sleeve gastrectomy: A meta-analysis. Surg. Obes. Relat. Dis. 2018, 14, 741–747. [Google Scholar] [CrossRef]
- Thereaux, J.; Lesuffleur, T.; Païta, M.; Czernichow, S.; Basdevant, A.; Msika, S.; Millat, B.; Fagot-Campagna, A. Long-term follow-up after bariatric surgery in a national cohort. Br. J. Surg. 2017, 104, 1362–1371. [Google Scholar] [CrossRef]
- Vidal, P.; Ramón, J.M.; Goday, A.; Parri, A.; Crous, X.; Trillo, L.; Pera, M.; Grande, L. Lack of adherence to follow-up visits after bariatric surgery: Reasons and outcome. Obes. Surg. 2014, 24, 179–183. [Google Scholar] [CrossRef]
- Kedestig, J.; Stenberg, E. Loss to follow-up after laparoscopic gastric bypass surgery—A post hoc analysis of a randomized clinical trial. Surg. Obes. Relat. Dis. 2019, 15, 880–886. [Google Scholar] [CrossRef]
- McVay, M.A.; Friedman, K.E.; Applegate, K.L.; Portenier, D.D. Patient predictors of follow-up care attendance in Roux-en-Y gastric bypass patients. Surg. Obes. Relat. Dis. 2013, 9, 956–962. [Google Scholar] [CrossRef]
- Goldenshluger, A.; Elazary, R.; Cohen, M.J.; Goldenshluger, M.; Ben-Porat, T.; Nowotni, J.; Geraisi, H.; Amun, M.; Pikarsky, A.J.; Keinan-Boker, L. Predictors for Adherence to Multidisciplinary Follow-Up Care after Sleeve Gastrectomy. Obes. Surg. 2018, 28, 3054–3061. [Google Scholar] [CrossRef]
- Moroshko, I.; Brennan, L.; O’Brien, P. Predictors of Attrition in Bariatric Aftercare: A Systematic Review of the Literature. Obes. Surg. 2012, 22, 1640–1647. [Google Scholar] [CrossRef]
- Jennings, N.; Boyle, M.; Mahawar, K.; Balupuri, S.; Small, P. The relationship of distance from the surgical centre on attendance and weight loss after laparoscopic gastric bypass surgery in the United Kingdom. Clin. Obes. 2013, 3, 180–184. [Google Scholar] [CrossRef]
- Barka, I.; Sayedoff, P.; Garnier, N.; Cussac-Pillegand, C.; Barrat, C.; Bihan, H. Sociodemographic Factors Associated with Loss to Follow-Up After Bariatric Surgery. Obes. Surg. 2021, 31, 2701–2708. [Google Scholar] [CrossRef]
- Lara, M.D.; Baker, M.T.; Larson, C.J.; Mathiason, M.A.; Lambert, P.J.; Kothari, S.N. Travel distance, age, and sex as factors in follow-up visit compliance in the post-gastric bypass population. Surg. Obes. Relat. Dis. 2005, 1, 17–21. [Google Scholar] [CrossRef]
- Kim, H.J.; Madan, A.; Fenton-Lee, D. Does Patient Compliance with Follow-up Influence Weight Loss After Gastric Bypass Surgery? A Systematic Review and Meta-Analysis. Obes. Surg. 2014, 24, 647–651. [Google Scholar] [CrossRef]
- Luca, P.; Nicolas, C.; Marina, V.; Sarah, B.; Andrea, L. Where Are My Patients? Lost and Found in Bariatric Surgery. Obes. Surg. 2021, 31, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Meunier, H.; Le Roux, Y.; Fiant, A.L.; Marion, Y.; Bion, A.L.; Gautier, T.; Contival, N.; Lubrano, J.; Fobe, F.; Zamparini, M.; et al. Does the Implementation of Enhanced Recovery After Surgery (ERAS) Guidelines Improve Outcomes of Bariatric Surgery? A Propensity Score Analysis in 464 Patients. Obes. Surg. 2019, 29, 2843–2853. [Google Scholar] [CrossRef] [PubMed]
- Vallois, A.; Menahem, B.; Le Roux, Y.; Bion, A.L.; Meunier, H.; Gautier, T.; Contival, N.; Mulliri, A.; Lubano, J.; Parienti, J.-J.; et al. Revisional Roux-en-Y Gastric Bypass: A Safe Surgical Opportunity? Results of a Case-Matched Study. Obes. Surg. 2019, 29, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Pornet, C.; Delpierre, C.; Dejardin, O.; Grosclaude, P.; Launay, L.; Guittet, L.; Lang, T.; Launoy, G. Construction of an adaptable European transnational ecological deprivation index: The French version. J. Epidemiol. Community Health 2012, 66, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Launay, L.; Guillot, F.; Gaillard, D.; Medjkane, M.; Saint-Gérand, T.; Launoy, G.; Dejardin, O. Methodology for building a geographical accessibility health index throughout metropolitan France. PLoS ONE 2019, 14, e0221417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rijswijk, A.S.; van Olst, N.; Schats, W.; van der Peet, D.L.; van de Laar, A.W. What Is Weight Loss After Bariatric Surgery Expressed in Percentage Total Weight Loss (%TWL)? A Systematic Review. Obes. Surg. 2021, 31, 3833–3847. [Google Scholar] [CrossRef]
- Sjöström, L.; Narbro, K.; Sjöström, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Barlsson, B.; et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef] [Green Version]
- Christou, N.V.; Sampalis, J.S.; Liberman, M.; Look, D.; Auger, S.; McLean, A.P.H.; MacLean, L.D. Surgery Decreases Long-term Mortality, Morbidity, and Health Care Use in Morbidly Obese Patients. Ann. Surg. 2004, 240, 416–424. [Google Scholar] [CrossRef]
- Rives-Lange, C.; Rassy, N.; Carette, C.; Phan, A.; Barsamian, C.; Thereaux, J.; Moszkowicz, D.; Poghosyan, T.; Czernichow, S. Seventy years of bariatric surgery: A systematic mapping review of randomized controlled trials. Surg. Obes. Relat. Dis. 2022, 23, e13420. [Google Scholar] [CrossRef]
- Auge, M.; Menahem, B.; Savey, V.; Lee Bion, A.; Alves, A. Long-term complications after gastric bypass and sleeve gastrectomy: What information to give to patients and practitioners, and why? J. Visc. Surg. 2022, 159, 298–308. [Google Scholar] [CrossRef]
- Jaffiol, C.; Bringer, J.; Laplace, J.P.; Buffet, C.; Attali, C.; Bringer, J. Améliorer le suivi des patients après chirurgie bariatrique. Bull. Acad. Natl. Méd. 2017, 201, 973–982. [Google Scholar] [CrossRef]
- Spaniolas, K.; Kasten, K.R.; Celio, A.; Burruss, M.B.; Pories, W.J. Postoperative Follow-up After Bariatric Surgery: Effect on Weight Loss. Obes. Surg. 2016, 26, 900–903. [Google Scholar] [CrossRef]
- Effect of COVID-19 Lockdowns on Physical Activity, Eating Behavior, Body Weight and Psychological Outcomes in a Post-Bariatric Cohort. SpringerLink. Available online: https://link.springer.com/article/10.1007/s11695-022-06069-x (accessed on 29 May 2022).
- Brown, A.M.; Ardila-Gatas, J.; Yuan, V.; Devas, N.; Docimo, S.; Spaniolas, K.; Pryor, A.D. The Impact of Telemedicine Adoption on a Multidisciplinary Bariatric Surgery Practice During the COVID-19 Pandemic. Ann. Surg. 2020, 272, e306–e310. [Google Scholar] [CrossRef]
- Runfola, M.; Fantola, G.; Pintus, S.; Iafrancesco, M.; Moroni, R. Telemedicine Implementation on a Bariatric Outpatient Clinic During COVID-19 Pandemic in Italy: An Unexpected Hill-Start. Obes. Surg. 2020, 27, 5145–5149. [Google Scholar] [CrossRef]
- Vallois, A.; Menahem, B.; Alves, A. Is Laparoscopic Bariatric Surgery Safe and Effective in Patients over 60 Years of Age? An Updated Systematic Review and Meta-Analysis. Obes. Surg. 2020, 30, 5059–5070. [Google Scholar] [CrossRef]
- Pouchucq, C.; Menahem, B.; Le Roux, Y.; Bouvier, V.; Gardy, J.; Meunier, H.; Thomas, F.; Launoy, G.; Dejardin, O.; Alves, A. Are Geographical Health Accessibility and Socioeconomic Deprivation Associated with Outcomes Following Bariatric Surgery? A Retrospective Study in a High-Volume Referral Bariatric Surgical Center. Obes. Surg. 2022, 32, 1486–1497. [Google Scholar] [CrossRef]
- El Ansari, W.; Elhag, W. Weight Regain and Insufficient Weight Loss After Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps—A Scoping Review. Obes. Surg. 2021, 31, 1755–1766. [Google Scholar] [CrossRef]
- Lee Bion, A.; Le Roux, Y.; Alves, A.; Menahem, B. Bariatric revisional surgery: What are the challenges for the patient and the practitioner? J. Visc. Surg. 2021, 158, 38–50. [Google Scholar] [CrossRef]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple imputation by chained equations: What is it and how does it work? Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auge, M.; Dejardin, O.; Menahem, B.; Lee Bion, A.; Savey, V.; Launoy, G.; Bouvier, V.; Alves, A. Analysis of the Lack of Follow-Up of Bariatric Surgery Patients: Experience of a Reference Center. J. Clin. Med. 2022, 11, 6310. https://doi.org/10.3390/jcm11216310
Auge M, Dejardin O, Menahem B, Lee Bion A, Savey V, Launoy G, Bouvier V, Alves A. Analysis of the Lack of Follow-Up of Bariatric Surgery Patients: Experience of a Reference Center. Journal of Clinical Medicine. 2022; 11(21):6310. https://doi.org/10.3390/jcm11216310
Chicago/Turabian StyleAuge, Marie, Olivier Dejardin, Benjamin Menahem, Adrien Lee Bion, Véronique Savey, Guy Launoy, Véronique Bouvier, and Arnaud Alves. 2022. "Analysis of the Lack of Follow-Up of Bariatric Surgery Patients: Experience of a Reference Center" Journal of Clinical Medicine 11, no. 21: 6310. https://doi.org/10.3390/jcm11216310
APA StyleAuge, M., Dejardin, O., Menahem, B., Lee Bion, A., Savey, V., Launoy, G., Bouvier, V., & Alves, A. (2022). Analysis of the Lack of Follow-Up of Bariatric Surgery Patients: Experience of a Reference Center. Journal of Clinical Medicine, 11(21), 6310. https://doi.org/10.3390/jcm11216310





