Endoscopic Transluminal Stent Placement for Malignant Afferent Loop Obstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Devices
2.3. Endoscopic Procedure
2.4. Data Collection
2.5. Definitions
2.6. Statistical Analysis
3. Results
3.1. Study Flow
3.2. Initial Cohort
3.3. SEMS Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pannala, R.; Brandabur, J.J.; Gan, S.I.; Gluck, M.; Irani, S.; Patterson, D.J.; Ross, A.S.; Dorer, R.; Traverso, L.W.; Picozzi, V.J.; et al. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: Single-center, 14-year experience. Gastrointest. Endosc. 2011, 74, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Saka, M.; Morita, S.; Fukagawa, T.; Katai, H. Afferent loop obstruction after distal gastrectomy with Roux-en-Y reconstruction. World J. Surg. 2010, 34, 2389–2392. [Google Scholar] [CrossRef]
- Juan, Y.H.; Yu, C.Y.; Hsu, H.H.; Huang, G.S.; Chan, D.C.; Liu, C.H.; Tung, H.J.; Chang, W.C. Using multidetector-row CT for the diagnosis of afferent loop syndrome following gastroenterostomy reconstruction. Yonsei Med. J. 2011, 52, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.B.; Yoo, D.; Hwang, D.W.; Lee, J.H.; Kwon, J.; Hong, S.; Lee, J.W.; Youn, W.Y.; Hwang, K.; Kim, S.C. Comparative analysis of afferent loop obstruction between laparoscopic and open approach in pancreaticoduodenectomy. J. Hepatobiliary Pancreat. Sci. 2019, 26, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Shiomi, H.; Masuda, A.; Kobayashi, T.; Yamada, Y.; Kodama, Y. Clinical management for malignant afferent loop obstruction. World J. Gastrointest. Oncol. 2021, 13, 684–692. [Google Scholar] [CrossRef]
- Sato, Y.; Inaba, Y.; Murata, S.; Yamaura, H.; Kato, M.; Kawada, H.; Shimizu, Y.; Ishiguchi, T. Percutaneous drainage for afferent limb syndrome and pancreatic fistula via the blind end of the jejunal limb after pancreatoduodenectomy or bile duct resection. J. Vasc. Interv. Radiol. 2015, 26, 566–572. [Google Scholar] [CrossRef]
- Ole Paulsen, B.; Amulf Skjennald, M.; Magne Osnes, M. An endoscopic drainage procedure for afferent loop occlusion. Gastrointest. Endosc. 1987, 3, 125–126. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamada, I.; Matsuyama, M.; Sasahira, N. Enteral stent placement for malignant afferent loop obstruction by the through-the-scope technique using a short-type single-balloon enteroscope. Endosc. Int. Open 2018, 6, E806–E811. [Google Scholar] [CrossRef] [Green Version]
- Nakahara, K.; Okuse, C.; Matsumoto, N.; Suetani, K.; Morita, R.; Michikawa, Y.; Ozawa, S.; Hosoya, K.; Kobayashi, S.; Otsubo, T.; et al. Enteral metallic stenting by balloon enteroscopy for obstruction of surgically reconstructed intestine. World J. Gastroenterol. 2015, 21, 7589–7593. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Shiomi, H.; Iemoto, T.; Nakano, R.; Ikegawa, T.; Kobayashi, T.; Masuda, A.; Kodama, Y. Endoscopic Self-Expandable Metal Stent Placement for Malignant Afferent Loop Obstruction After Pancreaticoduodenectomy: A Case Series and Review. Clin. Endosc. 2020, 53, 491–496. [Google Scholar] [CrossRef]
- Kida, A.; Kido, H.; Matsuo, T.; Mizukami, A.; Yano, M.; Arihara, F.; Matsuda, K.; Ogawa, K.; Matsuda, M.; Sakai, A. Usefulness of endoscopic metal stent placement for malignant afferent loop obstruction. Surg. Endosc. 2020, 34, 2103–2112. [Google Scholar] [CrossRef]
- Sasaki, T.; Yoshida, S.; Isayama, H.; Narita, A.; Yamada, T.; Enomoto, T.; Sumida, Y.; Kyo, R.; Kuwai, T.; Tomita, M.; et al. Short-Term Outcomes of Colorectal Stenting Using a Low Axial Force Self-Expandable Metal Stent for Malignant Colorectal Obstruction: A Japanese Multicenter Prospective Study. J. Clin. Med. 2021, 10, 4936. [Google Scholar] [CrossRef]
- Okamoto, T.; Sasaki, T.; Yoshio, T.; Mori, C.; Mie, T.; Furukawa, T.; Yamada, Y.; Takeda, T.; Kasuga, A.; Matsuyama, M.; et al. Outcomes after partially covered self-expandable metal stent placement for recurrent duodenal obstruction. Surg. Endosc. 2022; in press. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Burdick, J.S.; Garza, A.A.; Magee, D.J.; Dykes, C.; Jeyarajah, R. Endoscopic management of afferent loop syndrome of malignant etiology. Gastrointest. Endosc. 2002, 55, 602–605. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, C.H.; Huh, J.H.; Park, J.Y.; Park, S.W.; Song, S.Y.; Chung, J.; Bang, S. Endoscopic management of afferent loop syndrome after a pylorus preserving pancreatoduodenecotomy presenting with obstructive jaundice and ascending cholangitis. Clin. Endosc. 2011, 44, 59–64. [Google Scholar] [CrossRef]
- Kida, A.; Matsuda, K.; Noda, Y. Endoscopic metallic stenting by double-balloon enteroscopy and its overtube for malignant gastrointestinal obstruction as palliative treatment. Dig. Endosc. 2013, 25, 550–555. [Google Scholar] [CrossRef]
- Sasaki, T.; Isayama, H.; Kogure, H.; Yamada, A.; Aoki, T.; Kokudo, N.; Koike, K. Double-balloon enteroscope-assisted enteral stent placement for malignant afferent-loop obstruction after Roux-en-Y reconstruction. Endoscopy 2014, 46, E541–E542. [Google Scholar] [CrossRef] [Green Version]
- Kwong, W.T.; Fehmi, S.M.; Lowy, A.M.; Savides, T.J. Enteral stenting for gastric outlet obstruction and aff erent limb syndrome following pancreaticoduodenectomy. Ann. Gastroenterol. 2014, 27, 413–417. [Google Scholar]
- Shugo, H.; Hodo, Y.; Yoneshima, M. Endoscopic metallic stent insertion for malignant afferent loop obstruction using balloon-assisted enteroscopy: A case report. Am. J. Gastroenterol. 2015, 110, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Shiomi, H.; Okabe, Y.; Yagi, Y.; Kobayashi, T.; Shiomi, Y.; Takenaka, M.; Hoshi, N.; Arisaka, Y.; Kutsumi, H.; et al. Effectiveness of endoscopic self-expandable metal stent placement for afferent loop obstruction caused by pancreatic cancer recurrence after pancreaticoduodenectomy. Clin. J. Gastroenterol. 2015, 8, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Ishiyama, S.; Saito, H.; Ito, M.; Fujiwara, A.; Niguma, T.; Yoshioka, M.; Shiode, J. Metallic stent insertion with double-balloon endoscopy for malignant afferent loop obstruction. World J. Gastrointest. Endosc. 2015, 7, 665–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Hao, S.; Yang, F.; Di, Y.; Yao, L.; Li, J.; Jiang, Y.; Zhong, L.; Fu, D.; Jin, C. Endoscopic metal enteral stent placement for malignant afferent loop syndrome after pancreaticoduodenectomy. Videosurgery Other Miniinvasive Tech. 2015, 10, 257–265. [Google Scholar] [CrossRef]
- Nakahara, K.; Sato, Y.; Suetani, K.; Morita, R.; Michikawa, Y.; Kobayashi, S.; Itoh, F. Endoscopic Double Metallic Stenting in the Afferent and Efferent Loops for Malignant Afferent Loop Obstruction with Billroth II Anatomy. Clin. Endosc. 2016, 49, 97–99. [Google Scholar] [CrossRef]
- Shimatani, M.; Takaoka, M.; Tokuhara, M.; Kato, K.; Miyoshi, H.; Ikeura, T.; Okazaki, K. Through-the-scope self-expanding metal stent placement using newly developed short double-balloon endoscope for the effective management of malignant afferent-loop obstruction. Endoscopy 2016, 48, E6–E7. [Google Scholar] [CrossRef] [Green Version]
- Minaga, K.; Kitano, M.; Takenaka, M. Through-the-scope enteral metal stentplacement using a short-type single-balloonenteroscope for malignant surgicallyreconstructed jejunal stenosis (with video). Dig. Endosc. 2016, 28, 755–759. [Google Scholar] [CrossRef]
- Kanno, Y.; Ohira, T.; Harada, Y.; Koike, Y.; Yamagata, T.; Tanaka, M.; Shimada, T.; Ito, K. Metal Stent Placement in the Afferent Loop Obstructed by Peritoneal Metastases—Experience of Five Cases. Clin. Endosc. 2018, 51, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Abe, N.; Kondou, E.; Tsurumi, M.; Hashimoto, Y.; Ooki, A.; Nagao, G.; Masaki, T.; Mori, T.; Sugiyama, M. Endoscopic self-expandable metal stent placement for malignant afferent loop obstruction caused by peritoneal recurrence after total gastrectomy. Int. Cancer Conf. J. 2018, 7, 98–102. [Google Scholar] [CrossRef]
- Yane, K.; Katanuma, A.; Hayashi, T.; Takahashi, K.; Kin, T.; Nagai, K.; Tanaka, K.; Komatsu, N.; Endo, M.; Kobayashi, Y.; et al. Enteral self-expandable metal stent placement for malignant afferent limb syndrome using single-balloon enteroscope: Report of five cases. Endosc. Int. Open 2018, 6, E1330–E1335. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Shimatani, M.; Masuda, M.; Nakamaru, K.; Mitsuyama, T.; Fukata, N.; Ikeura, T.; Takaoka, M.; Okazaki, K.; Naganuma, M. Efficacy and safety of endoscopic stent placement for afferent loop obstruction using a short double-balloon endoscopy. DEN open 2022, 3, e154. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niu, H.; Yang, C.; Liu, J.; Wang, Y.; Ju, S.; Bai, Y.; Ren, J.; Xiong, B. Covered vs. uncovered self-expandable metal stents for palliation of malignant afferent loop obstruction: A multicenter study. Scand. J. Gastroenterol. 2022, 57, 364–370. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, N.; Itoi, T.; Tsuchiya, T.; Nagakawa, Y.; Tsuchida, A. One-step EUS-guided gastrojejunostomy with use of lumen-apposing metal stent for afferent loop syndrome treatment. Gastrointest. Endosc. 2015, 82, 166. [Google Scholar] [CrossRef]
- Shah, A.; Khanna, L.; Sethi, A. Treatment of afferent limb syndrome: Novel approach with endoscopic ultrasound-guided creation of a gastrojejunostomy fistula and placement of lumen-apposing stent. Endoscopy 2015, 47, E309–E310. [Google Scholar] [CrossRef] [Green Version]
- Taunk, P.; Cosgrove, N.; Loren, D.E.; Kowalski, T.; Siddiqui, A.A. Endoscopic ultrasound-guided gastroenterostomy using a lumen-apposing self-expanding metal stent for decompression of afferent loop obstruction. Endoscopy 2015, 47, E395–E396. [Google Scholar] [CrossRef]
- Ratone, J.P.; Caillol, F.; Bories, E.; Pesenti, C.; Godat, S.; Giovannini, M. Hepatogastrostomy by EUS for malignant afferent loop obstruction after duodenopancreatectomy. Endosc. Ultrasound 2015, 4, 250–252. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Pinto, E.; Grimm, I.S.; Baron, T.H. Efficacy of Endoscopically Created Bypass Anastomosis in Treatment of Afferent Limb Syndrome: A Single-Center Study. Clin. Gastroenterol. Hepatol. 2016, 14, 633–637. [Google Scholar] [CrossRef]
- Tyberg, A.; Perez-Miranda, M.; Sanchez-Ocana, R.; Penas, I.; de la Serna, C.; Shah, J.; Binmoeller, K.; Gaidhane, M.; Grimm, I.; Baron, T.; et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: A multicenter, international experience. Endosc. Int. Open 2016, 4, E276–E281. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Tsuchiya, T.; Tanaka, R.; Mitsuyoshi, H.; Mukai, S.; Nagakawa, Y.; Itoi, T. Afferent loop syndrome treated by endoscopic ultrasound-guided gastrojejunostomy, using a lumen-apposing metal stent with an electrocautery-enhanced delivery system. Endoscopy 2017, 49, E270–E272. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, O.I.B.; Irani, S.S.; Ngamruengphong, S.; Aridi, H.D.; Kunda, R.; Siddiqui, A.; Dollhopf, M.; Nieto, J.; Chen, Y.-I.; Sahar, N.; et al. Endoscopic ultrasound-guided entero-enterostomy for the treatment of afferent loop syndrome: A multicenter experience. Endoscopy 2018, 50, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Benallal, D.C.; Hoibian, S.; Caillol, F.; Bories, E.; Presenti, C.; Ratone, J.P.; Giovannini, M. EUS-guided gastroenterostomy for afferent loop syndrome treatment stent. Endosc. Ultrasound 2018, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Hijioka, S.; Maruki, Y.; Ohba, A.; Nagashio, Y.; Okusaka, T.; Saito, Y. Endoscopic ultrasound-guided gastroenterostomy using a metal stent for the treatment of afferent loop syndrome. Endoscopy 2019, 51, E153–E155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajin, M.; Catalano, M.F.; Khan, N.M.; Lajin, I. Endoscopic ultrasound-guided gastrojejunostomy using a 2-cm lumen-apposing metal stent to treat benign afferent loop syndrome. Endoscopy 2019, 51, 695–696. [Google Scholar] [CrossRef] [Green Version]
- Shiomi, H.; Kobayashi, T.; Sakai, A.; Shiomi, Y.; Masuda, A.; Bondoc, E.M.; Kodama, Y. Endoscopic ultrasound-guided gastrojejunostomy using fully covered metal stent combined with large-loop double-pigtail stent for malignant afferent loop syndrome. Endoscopy 2019, 51, E303–E304. [Google Scholar] [CrossRef] [Green Version]
- El Bacha, H.; Leblanc, S.; Bordacahar, B.; Brieau, B.; Barret, M.; Savier, E.; Soubrane, O.; Dousset, B.; Prat, F. Endoscopic Ultrasound-Guided Enteroenterostomy for Afferent Limb Syndrome. ACG Case Rep. J. 2020, 7, e00442. [Google Scholar] [CrossRef]
- Mandai, K.; Uno, K.; Yasuda, K. Endoscopic ultrasound-guided treatment for malignant afferent loop obstruction after Roux-en-Y reconstruction. DEN Open 2020, 1, e3. [Google Scholar] [CrossRef]
- Ligresti, D.; Amata, M.; Messina, M.; Traina, M.; Tarantino, I. Single-step EUS-guided jejunojejunostomy with a lumen-apposing metal stent as treatment for malignant afferent limb syndrome. VideoGIE 2020, 5, 154–156. [Google Scholar] [CrossRef]
- De Bie, C.; Bronswijk, M.; Vanella, G.; Perez-Cuadrado-Robles, E.; van Malenstein, H.; Laleman, W.; Van der Merwe, S. EUS-guided hepaticogastrostomy for patients with afferent loop syndrome: A comparison with EUS-guided gastroenterostomy or percutaneous drainage. Surg. Endosc. 2022, 36, 2393–2400. [Google Scholar] [CrossRef]
- Jonason, D.; Amateau, S.K.; Freeman, M.L.; Trikudanathan, G. Endoscopic ultrasound- and fluoroscopy-guided jejunojejunostomy with a lumen-apposing metal stent for malignant afferent loop obstruction. Endoscopy 2022, 54, E602–E603. [Google Scholar] [CrossRef]
- Tanikawa, T.; Urata, N.; Ishii, K.; Katsumata, R.; Nishino, K.; Suehiro, M.; Kawanaka, M.; Haruma, K.; Kawamoto, H. Afferent-Loop Syndrome Treated with Endoscopic Ultrasound-Guided Drainage of the Afferent Loop with a Plastic Stent. Case Rep. Gastroenterol. 2022, 16, 122–128. [Google Scholar] [CrossRef] [PubMed]
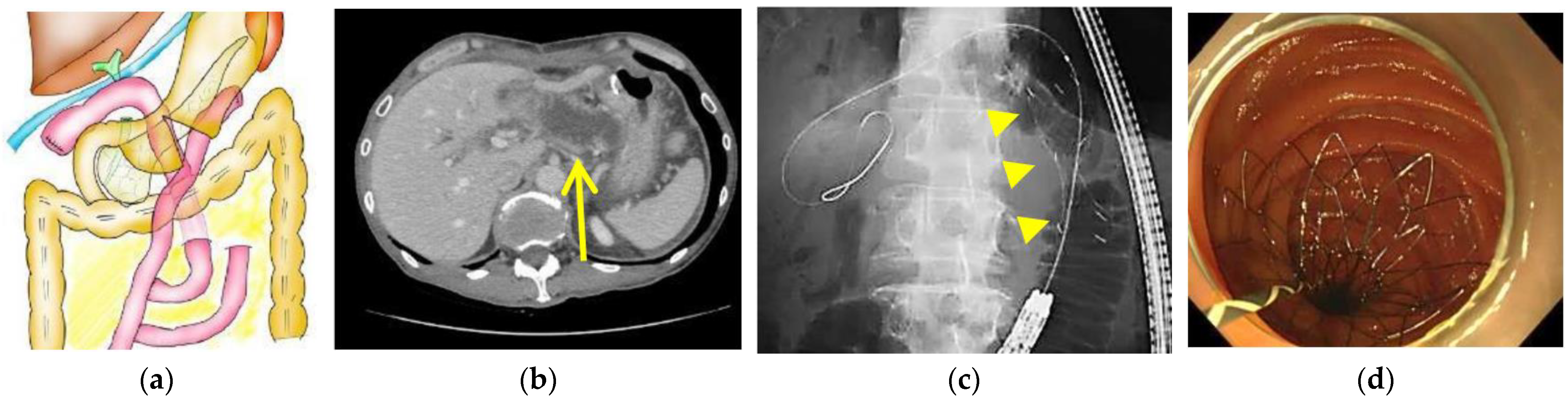

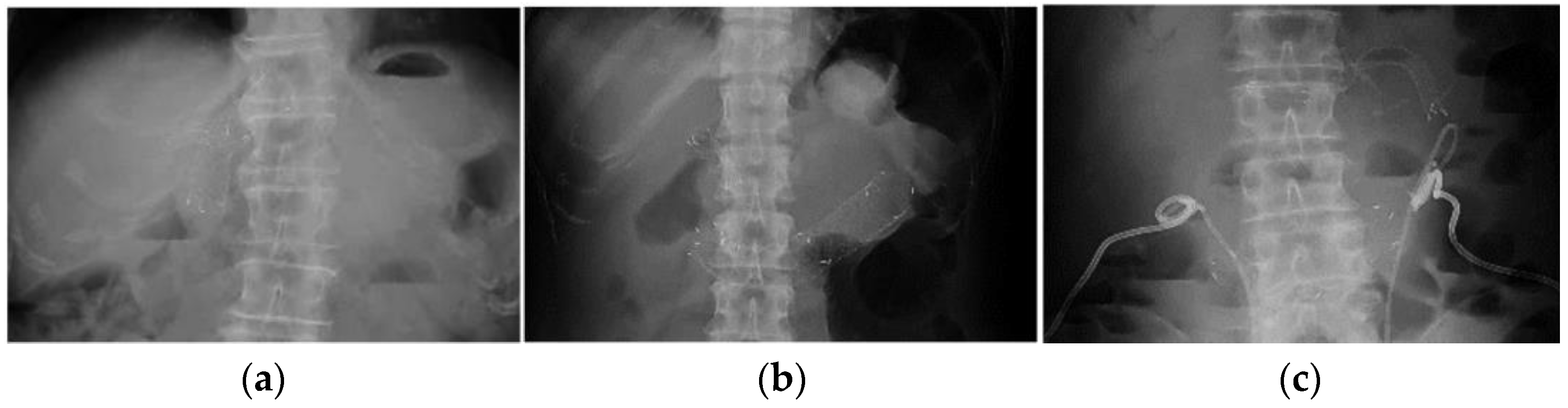
| Age, Years, Median (Range) | 64.5 (37–83) |
| Sex, n (%) | |
| Male | 13 (59.1%) |
| Female | 9 (40.9%) |
| Eastern Cooperative Oncology Group performance status, n (%) | |
| 0 | 10 (45.5%) |
| 1 | 8 (36.4%) |
| 2 | 4 (18.2%) |
| Primary disease, n (%) | |
| Pancreatic cancer | 10 (45.5%) |
| Biliary tract cancer | 6 (27.3%) |
| Gastric cancer | 5 (22.7%) |
| Esophageal cancer | 1 (4.5%) |
| Type of surgery, n (%) | |
| Open | 21 (95.5%) |
| Laparoscopic | 1 (4.5%) |
| Type of reconstruction, n (%) | |
| Pancreaticoduodenectomy | 11 (50.0%) |
| Gastrectomy with Roux-en-Y reconstruction | 6 (27.3%) |
| Hepatectomy with Roux-en-Y reconstruction | 3 (13.6%) |
| Double bypass | 1 (4.5%) |
| Unknown (before the procedure) | 1 (4.5%) |
| Ascites, n (%) | 12 (54.5%) |
| Dilation of intrahepatic bile duct, n (%) | 5 (22.7%) |
| Obstruction type, n (%) | |
| 1 | 15 (68.2%) |
| 2 | 4 (18.2%) |
| 3 | 3 (13.6%) |
| Symptoms of ALO, n (%) * | |
| Abdominal pain | 11 (50.0%) |
| Fever | 9 (40.9%) |
| Elevated hepatobiliary enzymes or jaundice | 4 (18.2%) |
| Nausea and vomiting | 1 (4.5%) |
| Time from surgery to ALO, days, median (range) | 612 (86–1806) |
| Use of antithrombotic agents, n (%) | 5 (22.7%) |
| Prior treatment with anti-vascular endothelial growth factor antibody, n (%) | 1 (4.5%) |
| Prior radiotherapy, n (%) | 1 (4.5%) |
| Time to reaching the stenotic site, min, median (range) | 10.5 (4–64) |
| Total procedure time, min, median (range) | 28.0 (12–106) |
| Endoscopic passage through the stenotic site, n (%) | 7 (31.8%) |
| Drainage method, n (%) | |
| END | 15 (68.2%) |
| SEMS | 7 (31.8%) |
| Technical success, n (%) | 21 (95.5%) |
| Clinical success, n (%) | 21 (95.5%) |
| Early complications, n (%) | 1 (4.5%) |
| Micro-perforation | 1 (4.5%) |
| Bleeding | 0 (0.0%) |
| Stent migration | 0 (0.0%) |
| END before SEMS placement, n (%) | 11 (61.1%) |
| Length of the stricture, mm, median (range) | 23 (10–53) |
| Total procedure time, min, median (range) | 25.0 (14–66) |
| Technical success, n (%) | 18 (100%) |
| Clinical success, n (%) | 18 (100%) |
| Stent diameter, n (%) | |
| 18 mm | 4 (22.2%) |
| 22 mm | 14 (77.8%) |
| Stent length, n (%) | |
| 60 mm | 2 (11.1%) |
| 80 mm | 5 (27.8%) |
| 100 mm | 5 (27.8%) |
| 120 mm | 6 (33.3%) |
| Early complications, n (%) | 0 (0.0%) |
| Follow-up period, days, median (range) | 102 (41–549) |
| Late complications, n (%) | |
| Abscess around the SEMS | 1 (5.6%) |
| Stent migration | 0 (0.0%) |
| Recurrence of ALO, n (%) | 2 (11.1%) |
| Re-intervention, n (%) | 1 (5.6%) |
| Received chemotherapy after SEMS placement, n (%) | 11 (61.1%) |
| Time to recurrent obstruction, days, median (95% CI) | NA (119–NA) |
| Overall survival, days, median (95% CI) | 102 (62–180) |
| Authors | Year | n | Type of Reconstruction | Type of SEMS | TSR (%) | CSR (%) | EC (%) | TTS/OA, (n) | GIE/BAE (n) | Recurrent Obstruction Rate, (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| B-II/PD/RY/Other (n) | UC/C (n) | |||||||||
| Burdick et al. | 2002 | 1 | 1/0/0/0 | NA | 100 | 100 | 0 | NA | NA | NA |
| Kim et al. | 2011 | 2 | 0/2/0/0 | 2/0 | 100 | 100 | 0 | 2/0 | 2/0 | NA |
| Kida et al. | 2013 | 1 | 0/1/0/0 | NA | 100 | 100 | 0 | 0/1 | 0/1 | NA |
| Sasaki et al. | 2014 | 1 | 0/1/0/0 | 1/0 | 100 | 100 | 0 | 0/1 | 0/1 | NA |
| Kwong et al. | 2014 | 2 | 0/2/0/0 | 2/0 | 100 | 100 | 0 | NA | NA | 0 |
| Shugo et al. | 2015 | 1 | 0/1/0/0 | 1/0 | 100 | 100 | 0 | 0/1 | 1/0 | 0 |
| Sakai et al. | 2015 | 1 | 0/1/0/0 | 1/0 | 100 | 100 | 0 | 1/0 | 1/0 | 0 |
| Fujii et al. | 2015 | 2 | 0/1/1/0 | 2/0 | 100 | 100 | 0 | 0/2 | 0/2 | 0 |
| Huang et al. | 2015 | 3 | 0/3/0/0 | 3/0 | 100 | 100 | 0 | 3/0 | 3/0 | 0 |
| Nakahara et al. | 2015 | 3 | 0/2/1/0 | 3/0 | 100 | 100 | 0 | 0/3 | 0/3 | 0 |
| Nakahara et al. | 2016 | 1 | 1/0/0/0 | 1/0 | 100 | 100 | 0 | 1/0 | NA | 0 |
| Shimataniet al. | 2016 | 1 | 0/1/0/0 | 1/0 | 100 | 100 | 0 | 1/0 | 0/1 | NA |
| Minaga et al. | 2016 | 1 | 0/1/0/0 | 1/0 | 100 | 100 | 0 | 1/0 | 0/1 | NA |
| Kannno et al. | 2018 | 4 | 0/3/1/0 | 4/0 | 100 | 100 | 0 | 4/0 | 2/2 | 0 |
| Takeuchi et al. | 2018 | 1 | 0/0/1/0 | 1/0 | 100 | 100 | 0 | 1/0 | 1/0 | 0 |
| Sasaki et al. | 2018 | 5 | 0/4/1/0 | 5/0 | 100 | 100 | 0 | 4/0 | 0/5 | NA |
| Yane et al. | 2018 | 5 | 0/4/1/0 | 5/0 | 100 | 100 | 0 | 1/4 | 0/5 | 0 |
| Sakai et al. | 2020 | 7 | 0/7/0/0 | 7/0 | 100 | 100 | 0 | 7/0 | 7/0 | 0 |
| Kida et al. | 2020 | 11 * | 3/7/1/0 | 10/0 | 91 | 91 | 0 | 9/1 | 6/5 | 20.0% |
| Ito et al. | 2022 | 10 | 0/NA/NA/0 | 10/0 | 100 | 100 | 0 | 10/0 | 0/10 | 20.0% |
| Wang et al. | 2022 | 137 | NA/76/NA/0 | 72/65 | 100 | 95 | 10.9% | 137/0 | 137/0 | 38.0% |
| Our study | 18 | 0/10/7/1 ** | 18/0 | 100 | 100 | 0 | 18/0 | 0/18 | 11.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonekura, C.; Sasaki, T.; Mie, T.; Okamoto, T.; Takeda, T.; Furukawa, T.; Yamada, Y.; Kasuga, A.; Matsuyama, M.; Ozaka, M.; et al. Endoscopic Transluminal Stent Placement for Malignant Afferent Loop Obstruction. J. Clin. Med. 2022, 11, 6357. https://doi.org/10.3390/jcm11216357
Yonekura C, Sasaki T, Mie T, Okamoto T, Takeda T, Furukawa T, Yamada Y, Kasuga A, Matsuyama M, Ozaka M, et al. Endoscopic Transluminal Stent Placement for Malignant Afferent Loop Obstruction. Journal of Clinical Medicine. 2022; 11(21):6357. https://doi.org/10.3390/jcm11216357
Chicago/Turabian StyleYonekura, Chinatsu, Takashi Sasaki, Takafumi Mie, Takeshi Okamoto, Tsuyoshi Takeda, Takaaki Furukawa, Yuto Yamada, Akiyoshi Kasuga, Masato Matsuyama, Masato Ozaka, and et al. 2022. "Endoscopic Transluminal Stent Placement for Malignant Afferent Loop Obstruction" Journal of Clinical Medicine 11, no. 21: 6357. https://doi.org/10.3390/jcm11216357





