Self-Medication for the Treatment of Abdominal Cramps and Pain—A Real-Life Comparison of Three Frequently Used Preparations
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Products
2.2. Study Design
2.3. Data Analysis
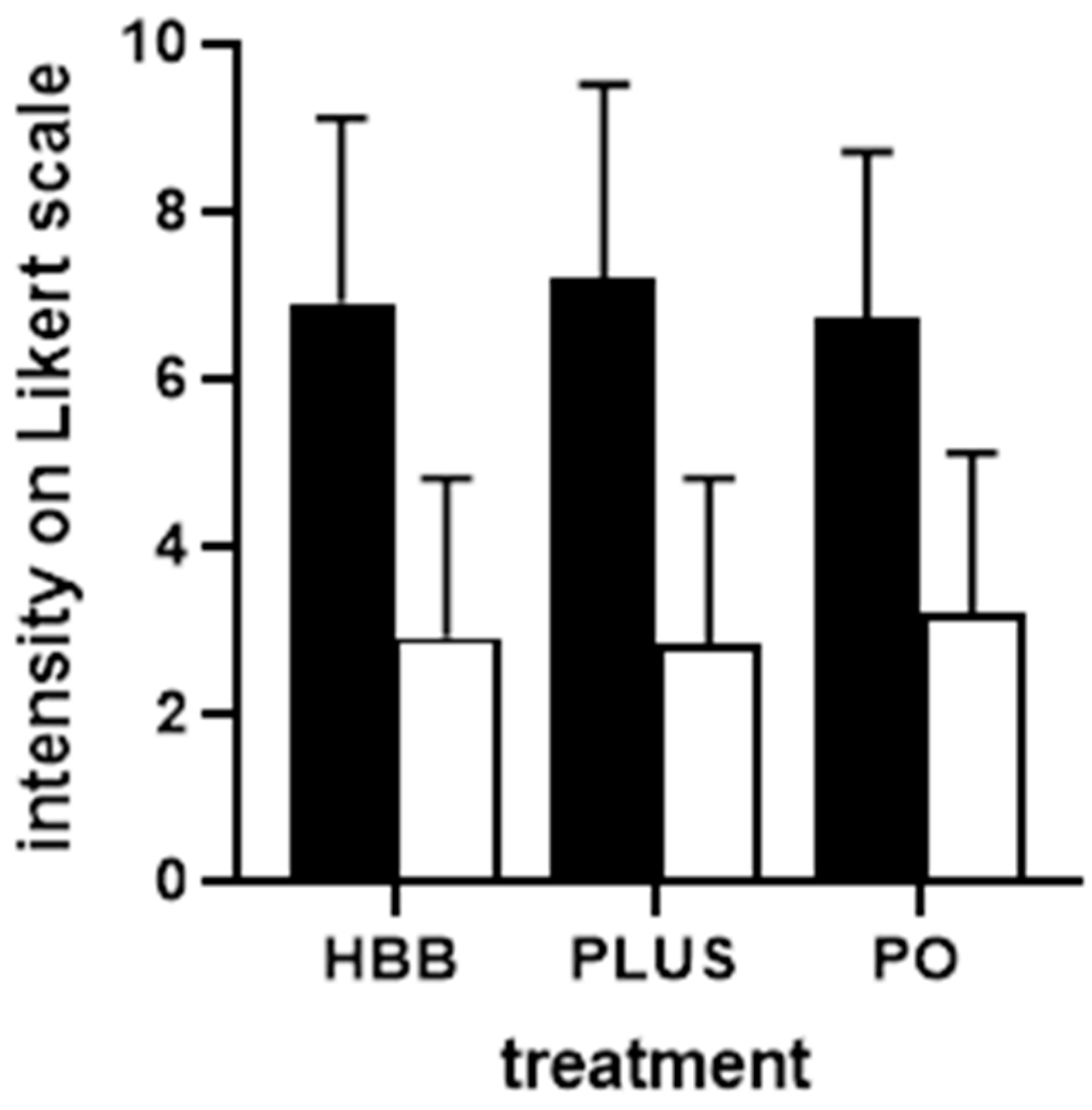
3. Results
3.1. Patient Characteristics
3.2. Pre-Treatment Symptoms
3.3. Treatment Outcomes
3.4. Post Hoc Logistic Regression Analyses
4. Discussion
4.1. Critique of Methods
4.2. Comparison of Populations
4.3. Perceived Effectiveness and Tolerability
4.4. Comparison of Preparations and Exploration of Other Explanatory Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandler, R.S.; Stewart, W.F.; Liberman, J.N.; Ricci, J.A.; Zorich, N.L. Abdominal pain, bloating, and diarrhea in the United States: Prevalence and impact. Dig. Dis. Sci. 2000, 45, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Bommelaer, G.; Poynard, T.; Le Pen, C.; Gaudin, A.F.; Maurel, F.; Priol, G.; Amouretti, M.; Frexinos, J.; Ruszniewski, P.; El Hasnaoui, A. Prevalence of irritable bowel syndrome (IBS) and variability of diagnostic criteria. Gastroenterol. Clin. Biol. 2004, 28 Pt 1, 554–561. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.E.; Van Tilburg, M.A.; Chang, L.; Chey, W.; Crowell, M.D.; Keefer, L.; Lembo, A.J.; Parkman, H.P.; Rao, S.S.; et al. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology 2016, 150, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Zeevenhooven, J.; Koppen, I.J.N.; Benninga, M.A. The new Rome IV criteria for functional gastrointestinal disorders in infants and toddlers. Pediatr. Gastroenterol. Hepatol. Nutr. 2017, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Mueller-Lissner, S.; Quigley, E.M.; Helfrich, I.; Schaefer, E. Drug treatment of chronic-intermittent abdominal cramping and pain: A multi-national survey on usage and attitudes. Aliment. Pharmacol. Ther. 2010, 32, 472–477. [Google Scholar] [CrossRef]
- Tytgat, G.N. Hyoscine butylbromide: A review of its use in the treatment of abdominal cramping and pain. Drugs 2007, 67, 1343–1357. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Mentha x piperita L., folium and Aetheroleum. 2020. Revision 1. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/european-union-herbal-monograph-mentha-x-piperita-l-folium-revision-1_en.pdf (accessed on 15 August 2022).
- Mueller-Lissner, S.; Tytgat, G.N.; Paulo, L.G.; Quigley, E.M.M.; Bubeck, J.; Peil, H.; Schaefer, E. Placebo- and paracetamol-controlled study on the efficacy and tolerability of hyoscine butylbromide in the treatment of patients with recurrent crampy abdominal pain. Aliment. Pharmacol. Ther. 2006, 23, 1741–1748. [Google Scholar] [CrossRef]
- Müller-Lissner, S.; Schäfer, E.; Kondla, A. Symptoms and their interpretation in patients self-treating abdominal cramping and pain with a spasmolytic (butylscoplolamine bromide)—A pharmacy-based survey. Pharmacol. Pharm. 2011, 2, 82–87. [Google Scholar] [CrossRef]
- Gaul, C.; Gräter, H.; Weiser, T. Results from a pharmacy-based patient survey on the use of a fixed combination analgesic containing acetylsalicylic acid, paracetamol and caffeine by self-diagnosing and self-treating patients. Springerplus 2016, 5, 721. [Google Scholar] [CrossRef]
- Klimek, L.; Schumacher, H.; Schütt, T.; Gräter, H.; Mück, T.; Michel, M.C. Factors associated with efficacy of an ibuprofen/pseudoephedrine combination drug in pharmacy customers with common cold symptoms. Int. J. Clin. Pract. 2017, 71, e12907. [Google Scholar] [CrossRef] [PubMed]
- Kardos, P.; Beeh, K.-M.; Sent, U.; Mueck, T.; Gräter, H.; Michel, M.C. Characterization of differential patient profiles and therapeutic responses of pharmacy customers for four ambroxol formulations. BMC Pharmacol. Toxicol. 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Gaul, C.; Gräter, H.; Weiser, T.; Michel, M.C.; Lampert, A.; Plomer, M.; Förderreuther, S. Impact of the neck and/or shoulder pain on self-reported headache treatment responses—Results from a pharmacy-based patient survey. Front. Neurol. 2022, 13, 902020. [Google Scholar] [CrossRef] [PubMed]
- Vollert, J.; Schenker, E.; Macleod, M.; Bespalov, A.; Wuerbel, H.; Michel, M.; Dirnagl, U.; Potschka, H.; Waldron, A.M.; Wever, K.; et al. Systematic review of guidelines for internal validity in the design, conduct and analysis of preclinical biomedical experiments involving laboratory animals. BMJ Open Sci. 2020, 4, e100046. [Google Scholar] [CrossRef]
- Michel, M.C.; Murphy, T.J.; Motulsky, H.J. New author guidelines for displaying data and reporting data analysis and statistical methods in experimental biology. Drug Metab. Dispos. 2020, 48, 64–74. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef]
- Miyoshi, A. A multi-centre, double-blind evaluation against placebo of the therapeutic effect of hyoscine-N-butylbromide in patients with abdominal pain. Pharmatherapeutica 1976, 1, 46–51. [Google Scholar]
- De Gregorio, M.; Damiani, S.; Gatta, G. Antalgic properties of proxazole. Double blind study in visceral algoplastic conditions. Panminerva Med. 1969, 11, 436–440. [Google Scholar]
- Neiger, A.; Bircher, J. Treatment of functional abdominal distress. Double-blind test with Buscopan and Tranquo-Buscopan. Schweiz Med. Wochenschr. 1970, 100, 1237–1240. [Google Scholar]
- Schäfer, E.; Ewe, K. The treatment of irritable colon. Efficacy and tolerance of buscopan plus, buscopan, paracetamol and placebo in ambulatory patients with irritable colon. Fortschr. Med. 1990, 108, 488–492. [Google Scholar]
- Ritchie, J.A.; Truelove, S.C. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br. Med. J. 1979, 1, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Yuan, Y.; Zhang, S.; Hou, X.; Wang, J.; Cai, J.; Shi, R.; Li, Y.; Wang, B.; Ji, F.; et al. Efficacy and tolerability of two oral hyoscine butylbromide formulations in Chinese patients with recurrent episodes of self-reported gastric or intestinal spasm-like pain. Int. J. Clin. Pharmacol. Ther. 2011, 49, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Poonai, N.; Kumar, K.; Coriolano, K.; Thompson, G.; Brahmbhatt, S.; Dzongowski, E.; Stevens, H.; Gupta, P.; Miller, M.; Elsie, S.; et al. Hyoscine butylbromide versus acetaminophen for nonspecific colicky abdominal pain in children: A randomized controlled trial. CMAJ 2020, 192, E1612–E1619. [Google Scholar] [CrossRef]
- Alammar, N.; Wang, L.; Saberi, B.; Nanavati, J.; Holtmann, G.; Shinohara, R.T.; Mullin, G.E. The impact of peppermint oil on the irritable bowel syndrome: A meta-analysis of the pooled clinical data. BMC Complement. Altern. Med. 2019, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Csoke, E.; Francis, M.; Ma, L.; Pohlhaus, D.; Landes, S.; Anquez-Traxler, C. How can real-world evidence aid decision making during the life cycle of non-prescription medicines? Clin. Transl. Sci. 2022, 15, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Göbel, H.; Gessner, U.; Petersen-Braun, M.; Weingärtner, U. Acetylsalicylic acid in self-medication of migraine. A pharmacy-based observational study. Schmerz 2007, 21, 49–54. [Google Scholar] [CrossRef]
- Watkins, C.; Tishkovskaya, S.; Brown, C.; Sutton, C.; Garcia, Y.S.; Forshaw, D.; Prescott, G.; Thomas, L.; Roffe, C.; Booth, J.; et al. Systematic voiding programme in adults with urinary incontinence following acute stroke: The ICONS-II RCT. Health Technol. Assess. 2022, 26, 1–88. [Google Scholar] [CrossRef]
- Eglen, R.M.; Reddy, H.; Watson, N.; Chaliss, R.A. Muscarinic acetylcholine receptor subtypes in smooth muscle. Trends Pharmacol. Sci. 1994, 15, 114–119. [Google Scholar] [CrossRef]
- Kearns, G.L.; Chumpitazi, B.P.; Abdel-Rahman, S.M.; Garg, U.; Shulman, R.J. Systemic exposure to menthol following administration of peppermint oil to paediatric patients. BMJ Open 2015, 5, e008375. [Google Scholar] [CrossRef]

| HBB | PLUS | PO | |
|---|---|---|---|
| Demographics | |||
| n | 579 | 641 | 466 |
| Age, years | 45.9 ± 18.6 | 43.8 ± 17.8 | 46.0 ± 16.2 |
| Gender, % female | 77.8 | 84.2 | 78.8 |
| Complaints in past 30 days | |||
| GI cramps and pain, number of days | 6.6 ± 6.2 | 5.9 ± 5.6 | 10.3 ± 7.6 |
| Bloating, number of days | 7.5 ± 7.7 | 6.1 ± 6.8 | 11.3 ± 8.4 |
| Flatulence, number of days | 4.8 ± 8.4 | 6.9 ± 7.8 | 11.7 ± 8.7 |
| Current complaints | |||
| GI cramps and pain, % | 85.8 | 59.9 | 60.5 |
| Urinary tract complaints | n.o. | 9.6 | n.o. |
| Dysmenorrhea | n.o. | 51.4 | n.o. |
| IBS | 22.5 | n.o. | 62.9 |
| Bloating | 37.4 | n.o. | 62.9 |
| Flatulence | 20.4 | n.o. | 49.6 |
| Other | 16.4 | 6.6 | 3.9 |
| Indication | ||||
|---|---|---|---|---|
| GI Cramps and Pain | IBS | Bloating | Flatulence | |
| Preparation | ||||
| HBB | −0.1562 [−0.4824; 0.1701] | reference group | reference group | reference group |
| PLUS | reference group | n.d. | n.d. | n.d. |
| PO | −0.5949 [−0.9697; −0.2202] | −0.7166 [−1.2096; −0.2237] | −0.2013 [−0.6088; 0.2062] | 0.0094 [−0.4829; 0.5016] |
| Gender | ||||
| Female | reference group | reference group | reference group | reference group |
| Male | −0.1785 [−0.5105; 0.1536] | −0.1465 [−0.6867; 0.37379 | 0.0015 [−0.4706; 0.4736] | −0.0488 [−0.5907; 0.4930] |
| Age | −0.0106 [−0.0186; −0.0025] | −0.0208 [−0.0348; −0.0068] | −0.0129 [−0.0255; 0.0004] | −0.0075 [−0.0215; 0.0065] |
| Baseline work/chore impairment | 0.0319 [−0.0608; 0.1245] | 0.0400 [−0.1176; 0.1976] | 0.0233 [−0.1222; 0.1688] | 0.0333 [−0.1285; 0.1952] |
| Baseline leisure impairment | 0.1757 [0.0788; 0.2706] | 0.2589 [0.0875; 0.1976] | 0.2272 [0.0732; 0.3811] | 0.2264 [0.0516; 0.4011] |
| Baseline sleep impairment | 0.073 [0.0236; 0.1227] | 0.1009 [0.0217; 0.1800] | 0.1123 [0.0374; 0.1872] | 0.0929 [0.0049; 0.1808] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storr, M.; Weigmann, H.; Landes, S.; Michel, M.C. Self-Medication for the Treatment of Abdominal Cramps and Pain—A Real-Life Comparison of Three Frequently Used Preparations. J. Clin. Med. 2022, 11, 6361. https://doi.org/10.3390/jcm11216361
Storr M, Weigmann H, Landes S, Michel MC. Self-Medication for the Treatment of Abdominal Cramps and Pain—A Real-Life Comparison of Three Frequently Used Preparations. Journal of Clinical Medicine. 2022; 11(21):6361. https://doi.org/10.3390/jcm11216361
Chicago/Turabian StyleStorr, Martin, Harald Weigmann, Sabine Landes, and Martin C. Michel. 2022. "Self-Medication for the Treatment of Abdominal Cramps and Pain—A Real-Life Comparison of Three Frequently Used Preparations" Journal of Clinical Medicine 11, no. 21: 6361. https://doi.org/10.3390/jcm11216361
APA StyleStorr, M., Weigmann, H., Landes, S., & Michel, M. C. (2022). Self-Medication for the Treatment of Abdominal Cramps and Pain—A Real-Life Comparison of Three Frequently Used Preparations. Journal of Clinical Medicine, 11(21), 6361. https://doi.org/10.3390/jcm11216361







