The Gupta Perioperative Risk for Myocardial Infarct or Cardiac Arrest (MICA) Calculator as an Intraoperative Neurologic Deficit Predictor in Carotid Endarterectomy
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Definitions
2.3. Surgical Technique
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
3.2. Multivariable Analysis and Confounding Variables/Factors
3.3. The MICA Score Predictive Power
4. Discussion
5. Conclusions
6. Key Points
- Question: What is the role of the National Surgical Quality Improvement Program (NSQIP) Gupta Perioperative Myocardial Infarct or Cardiac Arrest (MICA) risk calculator in predicting the risk of intraoperative neurologic deficits in patients undergoing carotid endarterectomy?
- Finding: A negative predictive value of 97% was reached and a MICA score of at least 8, together with obesity, were shown to be the two greatest discrimination factors in patients that developed intraoperative neurologic deficits.
- Meaning: MICA may have a role in stratifying patients according to their probability of developing intraoperative neurologic deficits.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MICA | Gupta Perioperative Risk for Myocardial Infarction or Cardiac Arrest |
| NSQIP | National Surgical Quality Improvement Program |
| ASA | American Society of Anesthesiologists |
| MACE | Major adverse cardiovascular event(s) |
| CEA | Carotid endarterectomy |
| IND | Intraoperative neurologic deficits |
| RA | Regional anaesthesia |
| MRA | Magnetic resonance angiography |
| CACC | Carotid artery cross-clamping |
| CKD | Chronic kidney disease |
| ROC | Receiver operating characteristic |
| AUROC | Area under the receiver operating characteristic curve |
| CRT | Classification and regression tree |
| SD | Standard deviation |
| IQR | Interquartile range |
| BMI | Body mass index |
| OR | Crude odds ratio(s) |
| aOR | Adjusted odds ratio(s) |
| CI | Confidence interval |
| NPV | Negative predictive value |
| STROBE | STrengthening the Reporting of OBservational studies in Epidemiology |
References
- Rerkasem, A.; Orrapin, S.; Howard, D.P.; Rerkasem, K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst. Rev. 2020, 2020, CD001081. [Google Scholar] [CrossRef]
- Naylor, A.R.; Ricco, J.B.; De Borst, G.J.; Debus, S.; De Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; I Fowkes, F.J.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Uno, M.; Takai, H.; Yagi, K.; Matsubara, S. Surgical Technique for Carotid Endarterectomy: Current Methods and Problems. Neurol. Med. Chir. 2020, 60, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.J. Carotid endarterectomy. Br. J. Anaesth. 2007, 99, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; E Anderson, N.; Barber, P.A. Neurological complications of carotid revascularisation. J. Neurol. Neurosurg. Psychiatry 2011, 83, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.; Camelière, L.; Felgueiras, R.; Berger, L.; Rerkasem, K.; Rothwell, P.M.; Touzé, E. Periprocedural Myocardial Infarction After Carotid Endarterectomy and Stenting: Systematic Review and Meta-Analysis. Stroke 2015, 46, 2843–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-Neves, A.; Fragão-Marques, M.; Rocha-Neves, J.; Gamas, L.; Oliveira-Pinto, J.; Cerqueira, A.; Andrade, J.; Fernando-Teixeira, J. The Impact of Neutrophil-Tolymphocyte Ratio and Plateletto-Lymphocyte Ratio in Carotid Artery Disease. Port. J. Card. Thorac. Vasc. Surg. 2021, 28, 45–51. [Google Scholar] [PubMed]
- Anzola, G.; Limoni, P.; Cavrini, G. Predictors of Carotid Clamping Intolerance during Endarterectomy That Would Be Wise to Apply to Stenting Procedures. Cerebrovasc. Dis. 2008, 26, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Montisci, R.; Sanfilippo, R.; Bura, R.; Branca, C.; Piga, M.; Saba, L. Status of the Circle of Willis and Intolerance to Carotid Cross-clamping During Carotid Endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Myrcha, P.; Lewczuk, A.; Jakuciński, M.; Kozak, M.; Siemieniuk, D.; Różański, D.; Koziorowski, D.; Woźniak, W. The Anatomy of the Circle of Willis Is Not a Strong Enough Predictive Factor for the Prognosis of Cross-Clamping Intolerance during Carotid Endarterectomy. J. Clin. Med. 2020, 9, 3913. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Neves, J.; Pereira-Macedo, J.; Ferreira, A.; Dias-Neto, M.; Andrade, J.P.; Mansilha, A.A. Impact of intraoperative neurologic deficits in carotid endarterectomy under regional anesthesia. Scand. Cardiovasc. J. 2021, 55, 180–186. [Google Scholar] [CrossRef]
- Gupta, P.K.; Gupta, H.; Sundaram, A.; Kaushik, M.; Fang, X.; Miller, W.J.; Esterbrooks, D.J.; Hunter, C.B.; Pipinos, I.I.; Johanning, J.M.; et al. Development and Validation of a Risk Calculator for Prediction of Cardiac Risk After Surgery. Circulation 2011, 124, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, T.; Smilowitz, N.R.; Xia, Y.; Berger, J. Cardiovascular Risk Scores to Predict Perioperative Stroke in Noncardiac Surgery. Stroke 2019, 50, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.; Ghahramani, M.; Harris, S.; Suchniak-Mussari, K.; Bedi, G.; Bulathsinghala, C.; Foy, A. The Myocardial Infarction and Cardiac Arrest Risk Calculator is an Accurate Discriminator of Major Adverse Events Following Elective Hip and Knee Surgery. Circulation 2015, 132 (Suppl. 3), A11686. [Google Scholar]
- Peterson, B.; Ghahramani, M.; Harris, S.; Suchniak-Mussari, K.; Bedi, G.; Bulathsinghala, C.; Foy, A. Usefulness of the Myocardial Infarction and Cardiac Arrest Calculator as a Discriminator of Adverse Cardiac Events After Elective Hip and Knee Surgery. Am. J. Cardiol. 2016, 117, 1992–1995. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoneham, M.; Thompson, J. Arterial pressure management and carotid endarterectomy. Br. J. Anaesth. 2009, 102, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, V.C.; Poon, C.C.M. Intraoperative neuromonitoring in major vascular surgery. Br. J. Anaesth. 2016, 117, ii13–ii25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha-Neves, J.P.; Pereira-Macedo, J.; Leite-Moreira, A.; Oliveira-Pinto, J.P.; Afonso, G.; Mourão, J.; Andrade, J.P.; Vaz, R.; Mansilha, A. Efficacy of near-infrared spectroscopy cerebral oximetry on detection of critical cerebral perfusion during carotid endarterectomy under regional anesthesia. Vasa 2020, 49, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Alves-Ferreira, J.; Rocha-Neves, J.; Dias-Neto, M.; Braga, S.F. Poor long-term outcomes after carotid endarterectomy: A retrospective analysis of two portuguese centers. Scand. Cardiovasc. J. 2019, 53, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Dasenbrock, H.H.; Smith, T.R.; Gormley, W.B.; Castlen, J.P.; Patel, N.J.; Frerichs, K.U.; Aziz-Sultan, M.A.; Du, R. Predictive Score of Adverse Events After Carotid Endarterectomy: The NSQIP Registry Carotid Endarterectomy Scale. J. Am. Heart Assoc. 2019, 8, e013412. [Google Scholar] [CrossRef] [PubMed]
- Carreira, M.; Duarte-Gamas, L.; Rocha-Neves, J.; Andrade, J.P.; Fernando-Teixeira, J. Management of The Carotid Artery Stenosis in Asymptomatic Patients. Rev. Port. Cir. Cardiotorac. Vasc. 2020, 27, 159–166. [Google Scholar] [PubMed]
- Hakl, M.; Michalek, P.; Sevcik, P.; Pavlíková, J.; Stern, M. Regional anaesthesia for carotid endarterectomy: An audit over 10 years. Br. J. Anaesth. 2007, 99, 415–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P. Gupta Perioperative Risk for Myocardial Infarction or Cardiac Arrest (MICA)—MDCalc.: Mdcalc.com. 2022. Available online: https://www.mdcalc.com/calc/4038/gupta-perioperative-risk-myocardial-infarction-cardiac-arrest-mica#pearls-pitfalls (accessed on 5 January 2022).
- Pereira-Neves, A.; Rocha-Neves, J.; Fragão-Marques, M.; Duarte-Gamas, L.; Jácome, F.; Coelho, A.; Cerqueira, A.; Andrade, J.P.; Mansilha, A. Red blood cell distribution width is associated with hypoperfusion in carotid endarterectomy under regional anesthesia. Surgery 2021, 169, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Kass, G.V. An Exploratory Technique for Investigating Large Quantities of Categorical Data. J. R. Stat. Soc. 1980, 29, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Piffaretti, G.; Tarallo, A.; Franchin, M.; Bacuzzi, A.; Rivolta, N.; Ferrario, M.; Ferraro, S.; Bossi, M.; Castelli, P.; Tozzi, M. Outcome Analysis of Carotid Cross-Clamp Intolerance during Carotid Endarterectomy under Locoregional Anesthesia. Ann. Vasc. Surg. 2017, 43, 249–257. [Google Scholar] [CrossRef]
- Wang, H.-F.; Wang, D.-M.; Wang, J.-J.; Wang, L.-J.; Lu, J.; Qi, P.; Hu, S.; Yang, X.-M.; Chen, K.-P. Extracranial Internal Carotid Artery Tortuosity and Body Mass Index. Front. Neurol. 2017, 8, 508. [Google Scholar] [CrossRef]
- Durup-Dickenson, M.; Nicolajsen, C.W.; Budtz-Lilly, J.; Laustsen, J.; Eldrup, N. Body Mass Index and Operating Times in Vascular Procedures. EJVES Short Rep. 2017, 35, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R.S.; Sidawy, A.N.; Amdur, R.L.; Macsata, R.A. Obesity is an Independent Risk Factor for Death and Cardiac Complications after Carotid Endarterectomy. J. Am. Coll. Surg. 2012, 214, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rovella, V.; Anemona, L.; Cardellini, M.; Scimeca, M.; Saggini, A.; Santeusanio, G.; Bonanno, E.; Montanaro, M.; Legramante, I.M.; Ippoliti, A.; et al. The role of obesity in carotid plaque instability: Interaction with age, gender, and cardiovascular risk factors. Cardiovasc. Diabetol. 2018, 17, 46. [Google Scholar] [CrossRef] [Green Version]
- Han, T.S.; Oh, M.K.; Kim, S.M.; Yang, H.J.; Lee, B.S.; Park, S.Y.; Lee, W.J. Relationship between Neck Length, Sleep, and Cardiovascular Risk Factors. Korean J. Fam. Med. 2015, 36, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, R.D.; Eliasziw, M.; Fox, A.J.; Rothwell, P.M.; Barnett, H.J.M. Angiographically Defined Collateral Circulation and Risk of Stroke in Patients With Severe Carotid Artery Stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke 2000, 31, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Song, B.; Cheng, B.; Wong, K.S.; Xu, Y.M.; Ho, S.S.Y.; Chen, X.Y. Compensatory patterns of collateral flow in stroke patients with unilateral and bilateral carotid stenosis. BMC Neurol. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Patel, J.; Desikan, S.; Chrencik, M.; Martinez-Delcid, J.; Caraballo, B.; Yokemick, J.; Gray, V.L.; Sorkin, J.D.; Cebral, J.; et al. Asymptomatic carotid artery stenosis is associated with cerebral hypoperfusion. J. Vasc. Surg. 2020, 73, 1611–1621.e2. [Google Scholar] [CrossRef]
- Pothof, A.B.; Soden, P.A.; Fokkema, M.; Zettervall, S.L.; Deery, S.E.; Bodewes, T.C.; de Borst, G.J.; Schermerhorn, M.L. The impact of contralateral carotid artery stenosis on outcomes after carotid endarterectomy. J. Vasc. Surg. 2017, 66, 1727–1734.e2. [Google Scholar] [CrossRef] [Green Version]
- Knappich, C.; Kuehnl, A.; Haller, B.; Salvermoser, M.; Algra, A.; Becquemin, J.-P.; Bonati, L.H.; Bulbulia, R.; Calvet, D.; Fraedrich, G.; et al. Associations of Perioperative Variables With the 30-Day Risk of Stroke or Death in Carotid Endarterectomy for Symptomatic Carotid Stenosis. Stroke 2019, 50, 3439–3448. [Google Scholar] [CrossRef]
- de Borst, G.; Moll, F.; van de Pavoordt, H.; Mauser, H.; Kelder, J.; Ackerstaf, R. Stroke from carotid endarterectomy: When and how to reduce perioperative stroke rate? Eur. J. Vasc. Endovasc. Surg. 2001, 21, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Hicks, C.W.; Daya, N.R.; Black, J.H.; Matsushita, K.; Selvin, E. Race and sex-based disparities associated with carotid endarterectomy in the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2020, 292, 10–16. [Google Scholar] [CrossRef]


| Population n = 194 | Controls n = 97 | Cases of IND n = 97 | p-Value | |
|---|---|---|---|---|
| Gender | ||||
| Male, n (%) | 154 (79.4) | 81 (83.5) | 73 (75.2) | 0.089 |
| Female, n (%) | 40 (20.6) | 16 (16.6) | 24 (24.7) | |
| Age (years) | ||||
| Mean (±SD) | 70.2 (±9.0) | 69.0 (±8.6) | 71.4 (±9.2) | 0.065 |
| Median (IQR) | 71.5 (64–77) | 71.0 (62.0–75.8) | 72.0 (65.0–78.0) | |
| Side (right), n (%) | 98 (50.5) | 54 (55.7) | 44 (45.3) | 0.114 |
| CV risk factors, n (%) | ||||
| Hypertension | 171 (88.1) | 85 (87.6) | 86 (87.8) | 0.865 |
| Diabetes | 82 (42.3) | 38 (39.1) | 44 (44.9) | 0.454 |
| Smoking history | 100 (51.5) | 55 (56.7) | 45 (45.9) | 0.113 |
| Dyslipidaemia | 166 (85.6) | 82 (84.5) | 84 (86.5) | 0.953 |
| BMI > 30 kg /m2 | 35 (18.0) | 8 (8.2) | 27 (27.8) | <0.001 |
| CKD, n (%) | 25 (12.9) | 13 (13.4) | 12 (12.3) | 0.788 |
| PAD, n (%) | 47 (24.2) | 25 (25.7) | 22 (22.6) | 0.559 |
| CAD, n (%) | 70 (36.1) | 33 (34.0) | 37 (38.1) | 0.624 |
| COPD, n (%) | 24 (12.4) | 14 (14.4) | 10 (10.3) | 0.354 |
| CHF, n (%) | 25 (12.9) | 12 (12.3) | 13 (13.4) | 0.874 |
| AF, n (%) | 14 (7.3) | 7 (7.2) | 7 (7.2) | 0.984 |
| ASA, n (%) | ||||
| II | 31 (16.0) | 17 (17.5) | 14 (14.4) | |
| III | 150 (77.3) | 74 (76.2) | 76 (78.3) | 0.610 |
| IV | 13 (6.7) | 7 (7.2) | 6 (6.1) | |
| Functional status, n (%) | ||||
| Independent | 164 (84.5) | 85 (87.6) | 79 (1.4) | |
| Partially dependent | 24 (12.4) | 9 (9.3) | 15 (15.4) | 0.306 |
| Dependent | 6 (3.1) | 4 (4.1) | 2 (2.1) | |
| Asymptomatic, n (%) | 106 (54.6) | 53 (55.2) | 53 (54.1) | 0.875 |
| Symptomatic, n (%) | ||||
| TIA | 19 (9.8) | 10 (10.3) | 9 (9.3) | 0.922 |
| Stroke | 69 (35.6) | 33 (34.0) | 36 (37.1) | |
| Stenosis degree | ||||
| Mean (±SD) (%) | 83.8 ± 10.5 | 83.6 ± 9.6 | 82.1 ± 11.1 | 0.020 |
| Median (IQR) (%) | 80.0 (80–90) | 90.0 (80–90) | 70.0 (70–90) | |
| Contralateral stenosis degree | ||||
| Mean (±SD) (%) | 63.1 ± 19.0 | 59.6 ± 15.2 | 66.6 ± 21.7 | 0.012 |
| Median (IQR) (%) | 50.0 (50.0–70.0) | 50.0 (50.0–60.0) | 60.0 (50.0–80.0) | |
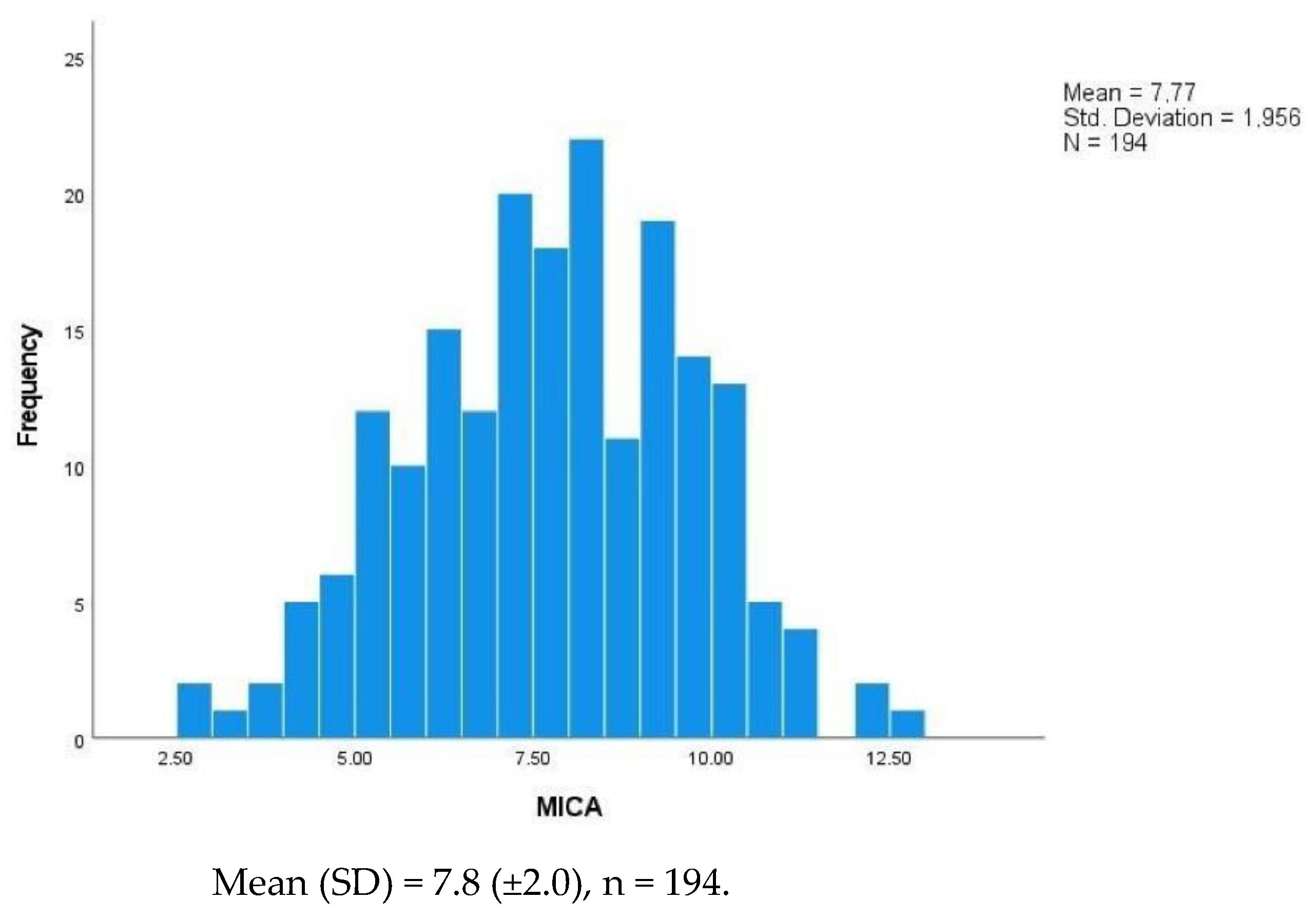
| MICA | ||||
| Mean (±SD) | 7.8 ± 2.0 | 7.5 ± 1.9 | 8.1 ± 2.0 | 0.032 |
| Median (IQR) | 7.8 (6.3–9.3) | 7.9 (6.7–9.5) | 7.7 (6.1–8.9) |
| Intraoperative Neurologic Deficit | ||||
|---|---|---|---|---|
| Crude OR (95%CI) | p-Value | aOR (95%CI) | p-Value | |
| Age | 1.03 (0.99–1.06) | 0.067 | - * | - |
| Sex (female) | 0.54 (0.27–1.10) | 0.091 | - | - |
| BMI > 30 kg/m2 | 4.18 (1.80–9.77) | 0.000 | 4.01 (1.66–9.67) | 0.002 |
| Ipsilateral stenosis | 0.72 (0.54–0.95) | 0.022 | 0.69 (0.51–0.94) | 0.019 |
| Contralateral stenosis | 1.22 (1.04–1.44) | 0.014 | 1.29 (1.08–1.53) | 0.004 |
| MICA score | 1.18 (1.01–1.36) | 0.034 | 1.21 (1.03–1.43) | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-Macedo, J.; Lopes-Fernandes, B.; Duarte-Gamas, L.; Pereira-Neves, A.; Mourão, J.; Khairy, A.; Andrade, J.P.; Marreiros, A.; Rocha-Neves, J. The Gupta Perioperative Risk for Myocardial Infarct or Cardiac Arrest (MICA) Calculator as an Intraoperative Neurologic Deficit Predictor in Carotid Endarterectomy. J. Clin. Med. 2022, 11, 6367. https://doi.org/10.3390/jcm11216367
Pereira-Macedo J, Lopes-Fernandes B, Duarte-Gamas L, Pereira-Neves A, Mourão J, Khairy A, Andrade JP, Marreiros A, Rocha-Neves J. The Gupta Perioperative Risk for Myocardial Infarct or Cardiac Arrest (MICA) Calculator as an Intraoperative Neurologic Deficit Predictor in Carotid Endarterectomy. Journal of Clinical Medicine. 2022; 11(21):6367. https://doi.org/10.3390/jcm11216367
Chicago/Turabian StylePereira-Macedo, Juliana, Beatriz Lopes-Fernandes, Luís Duarte-Gamas, António Pereira-Neves, Joana Mourão, Ahmed Khairy, José Paulo Andrade, Ana Marreiros, and João Rocha-Neves. 2022. "The Gupta Perioperative Risk for Myocardial Infarct or Cardiac Arrest (MICA) Calculator as an Intraoperative Neurologic Deficit Predictor in Carotid Endarterectomy" Journal of Clinical Medicine 11, no. 21: 6367. https://doi.org/10.3390/jcm11216367
APA StylePereira-Macedo, J., Lopes-Fernandes, B., Duarte-Gamas, L., Pereira-Neves, A., Mourão, J., Khairy, A., Andrade, J. P., Marreiros, A., & Rocha-Neves, J. (2022). The Gupta Perioperative Risk for Myocardial Infarct or Cardiac Arrest (MICA) Calculator as an Intraoperative Neurologic Deficit Predictor in Carotid Endarterectomy. Journal of Clinical Medicine, 11(21), 6367. https://doi.org/10.3390/jcm11216367






